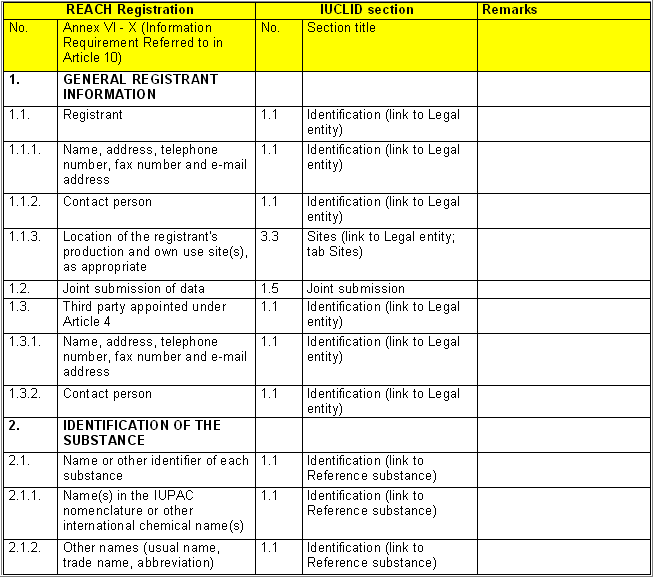
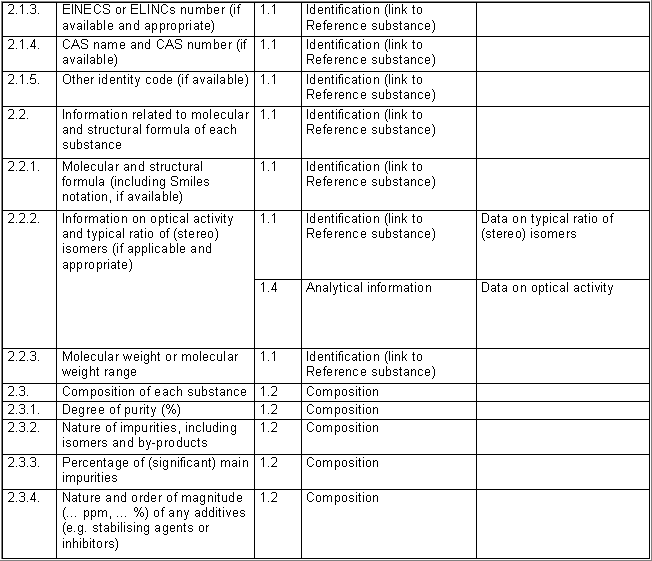
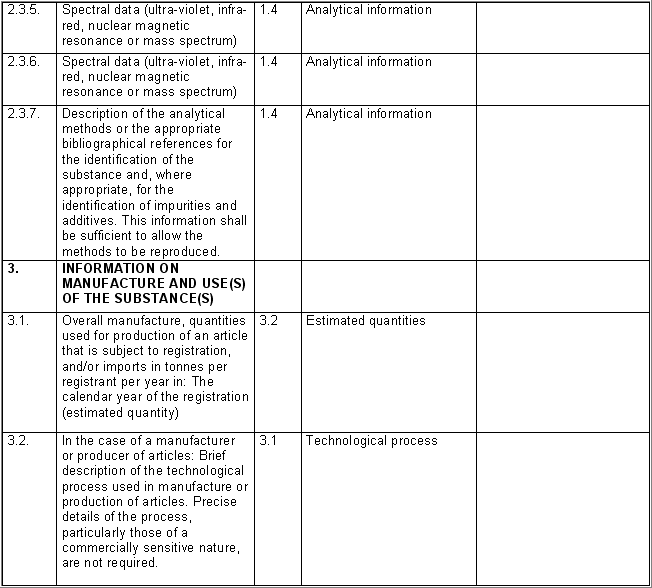
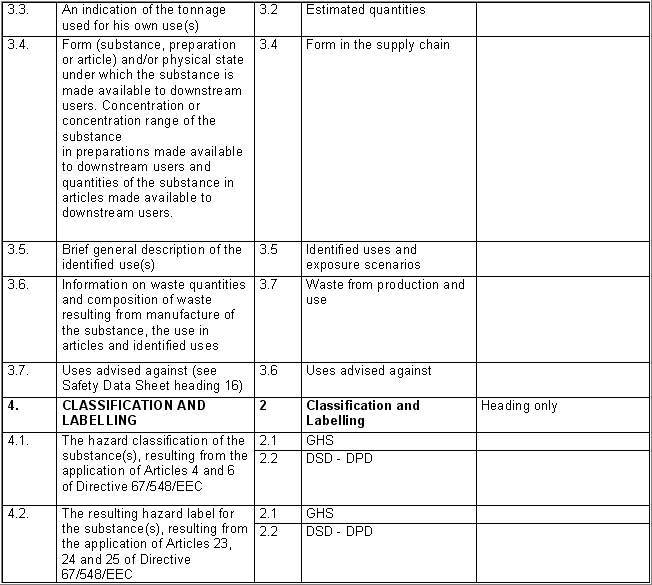
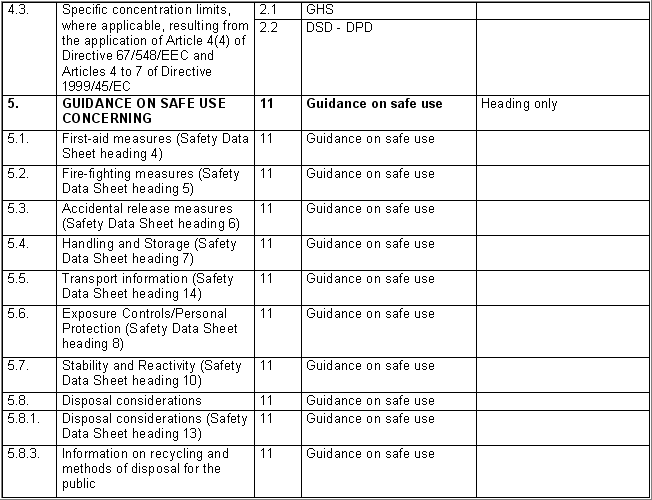
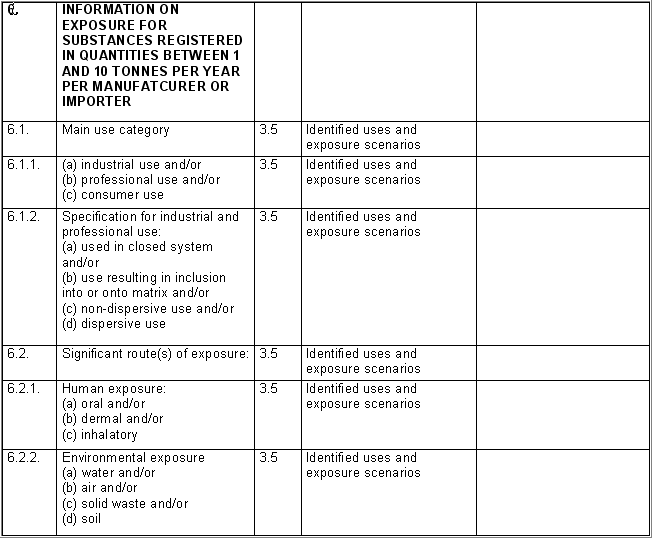
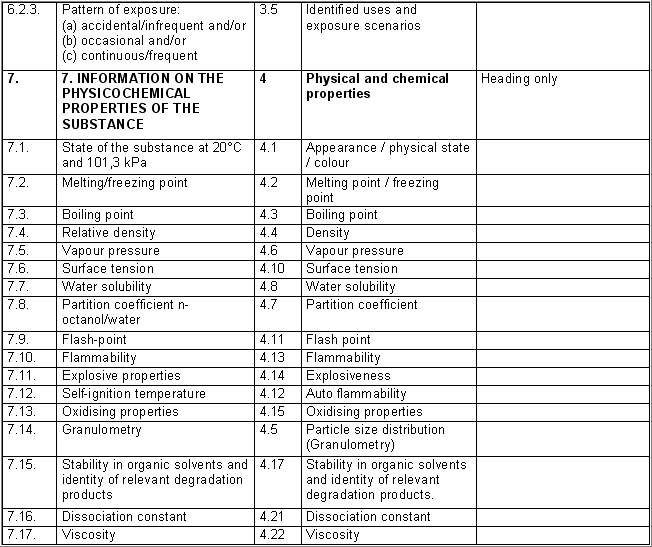
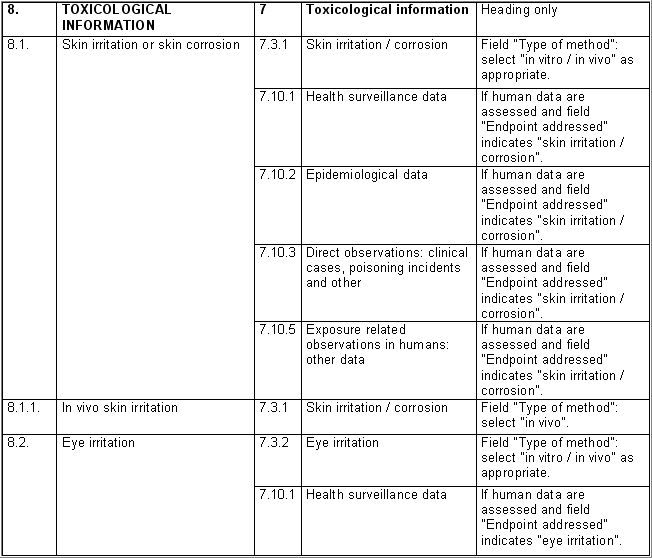
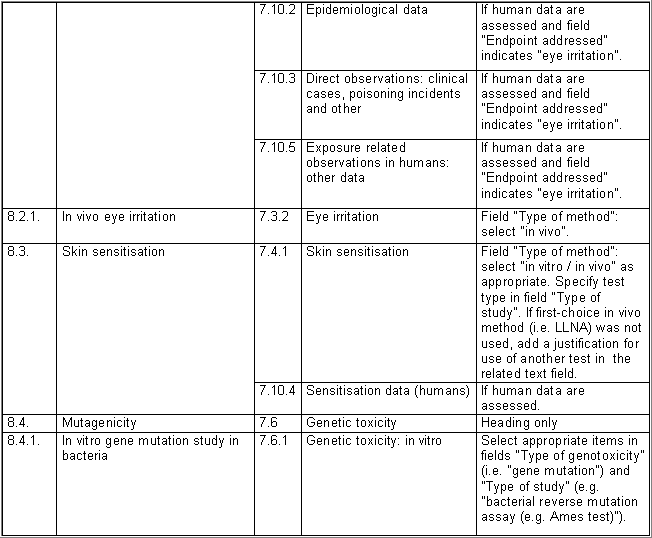
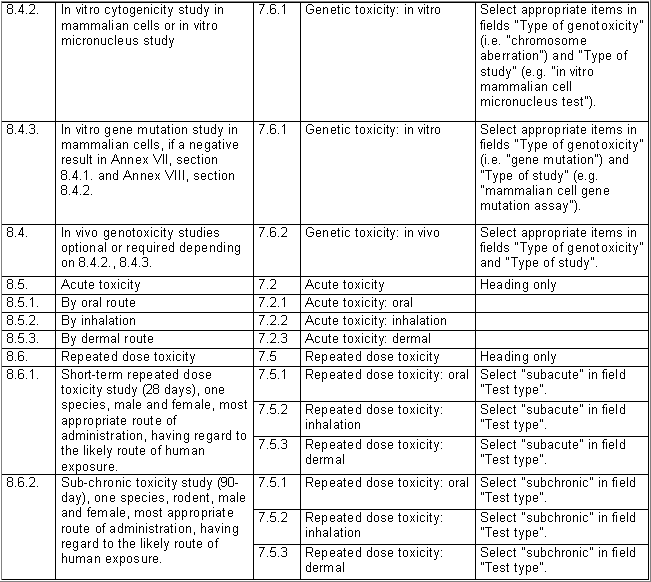
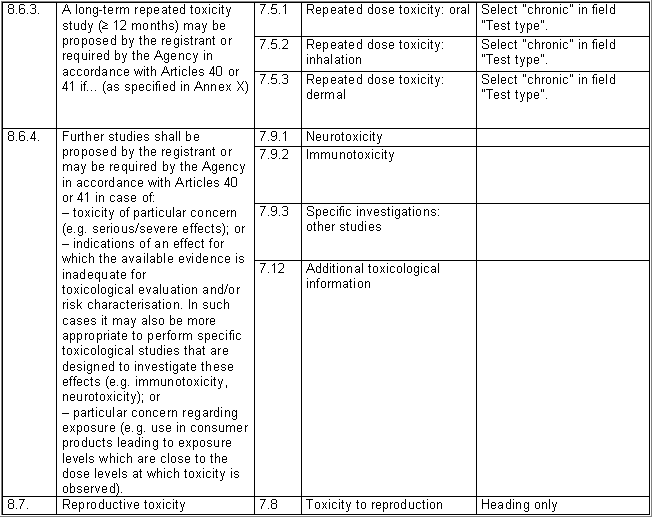
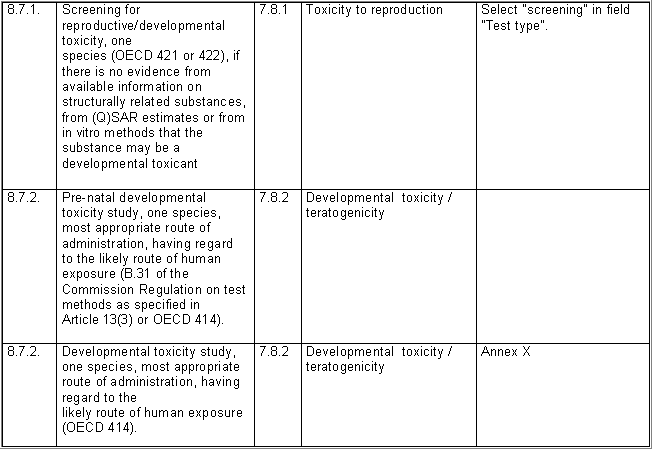
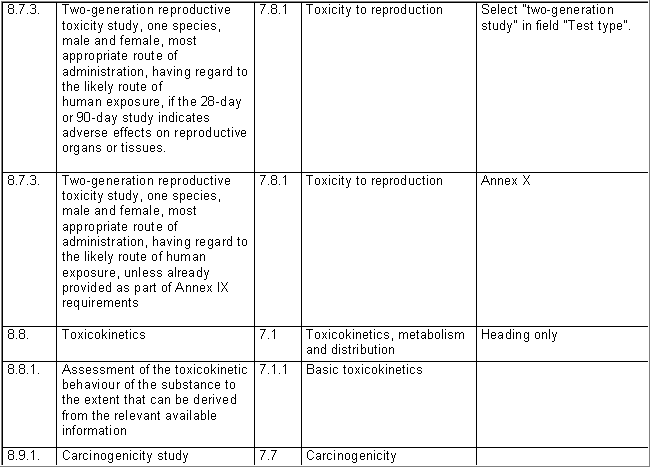
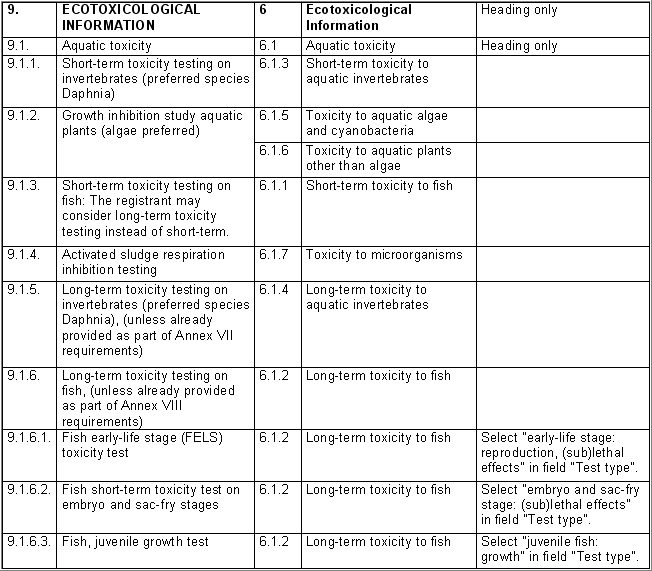
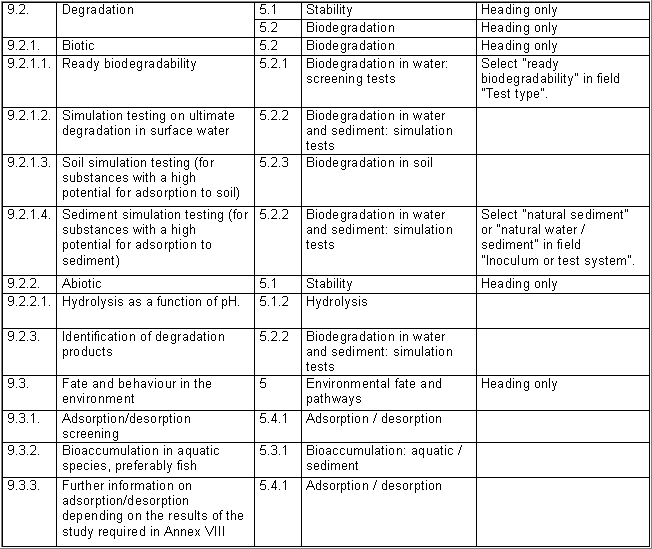
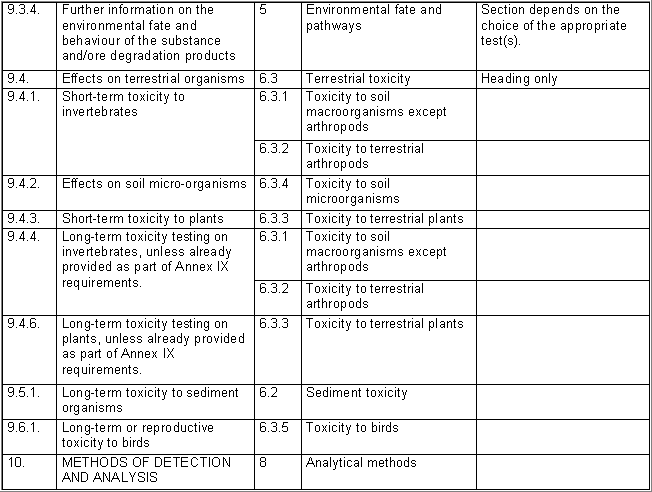
The following table specifies the REACH data points, i.e. the
information requirements as laid down in Annexes VI - X and referred to in
Article 10 of the REACH regulation, and the corresponding IUCLID sections
for recording the required data. In the Remarks column, hints are given as
to what specific data entry fields should be completed in order to specify
REACH data requirements. This is necessary when several REACH endpoints are
covered by one IUCLID section. For example, REACH data point 8.4
Mutagenicity includes three different types of genotoxicity test
systems, which are all recorded in IUCLID section 7.6.1 Genetic
toxicity: in vitro. The type of test system is documented in
fields Type of genotoxicity (e.g. "chromosome
aberration") and Type of study (e.g. "in vitro mammalian
cell micronucleus test").
Note
This specification table does not give any guidance on what data requirements apply for a given submission. Refer to the relevant REACH documentation thereof.