In the IUCLID Endpoint sections 4 to 10, Endpoint summary records can be created, which can be used to give an appraisal of all data compiled in a given Endpoint section. Hence, an endpoint summary addresses, in a very condensed form, the most relevant and reliable data. The summary may be confined to the highlights of one key study, if only one is available, or give a justification as to why the results of a given study are considered as key data in case several studies are available. The same holds true, where an endpoint requires a weight of evidence evaluation based on several studies.
This feature has been included in IUCLID in order to facilitate for the user the assessment of a chemical substance, particularly in the context of REACH, but also for other regulatory programmes where it is considered useful. In REACH, a registration dossier will consist of a technical dossier (IUCLID dossier) and a chemical safety report (CSR) attached to section 13 Assessment Reports, if required. An authority dossier is also foreseen as consisting of a technical dossier (IUCLID dossier) and an attachment (evaluation dossier, Annex XV dossier). Most of the "expert work" will be included in this attachment, i.e. the interpretation and use of the available data to prepare a CSR, derivation of DNEL and PNEC values, or within the processes of evaluation or preparation of an Annex XV dossier by the authorities.
The Endpoint summaries can be used
for the hazard assessment in the CSR
for C&L purposes (i.e. classification and labelling)
It is appropriate to complete the Endpoint summary records when the dataset provides sufficient data and before the Dossier is created. The information entered should focus on the most important data. If the relevant Endpoint study records contain executive summaries, extracting data from these may minimise duplication of work.
Endpoint summary records cannot only be created for Endpoint sections
(see chapter D.4.8.6.1 Summaries of individual
Endpoint sections), but also at a higher level, i.e. for main
sections, in which no endpoint data can be entered directly (see chapter D.4.8.6.2 Summaries at higher level) .
For instance, Endpoint sections 6.1.1 Short-term toxicity to
fish, 6.1.2 Long-term toxicity to fish,
6.1.3 Short-term toxicity to aquatic invertebrates,
etc, are grouped in section 6.1 Aquatic toxicity.
Endpoint summary records can be created for either of sections 6.1.1, 6.1.2,
6.1.3, etc., but also for the group section 6.1. The template for the latter
provides only one Discussion field, while the templates
for the sections subsumed thereunder include some discrete data entry
fields.
The design of the Endpoint summary records is rather uniform across all but a few Endpoint sections. Two examples are shown in the following screenshots:
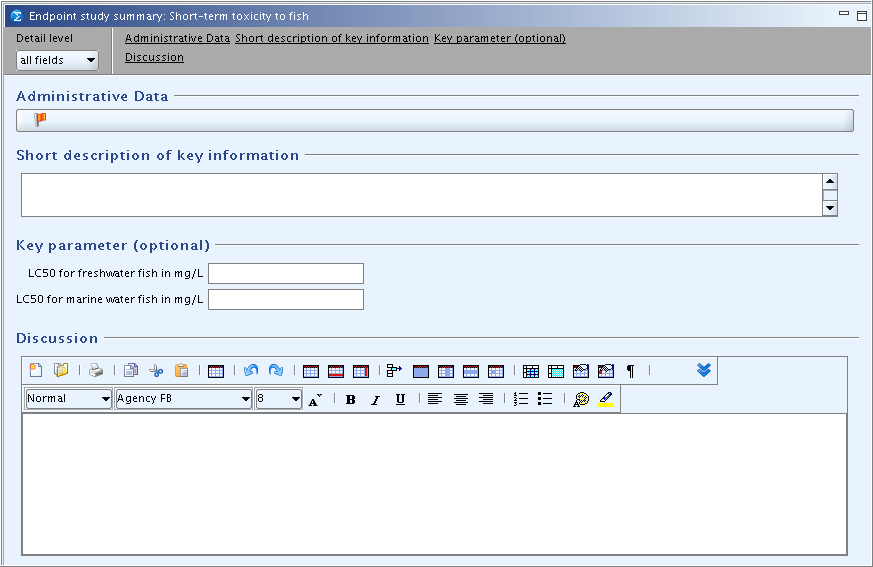
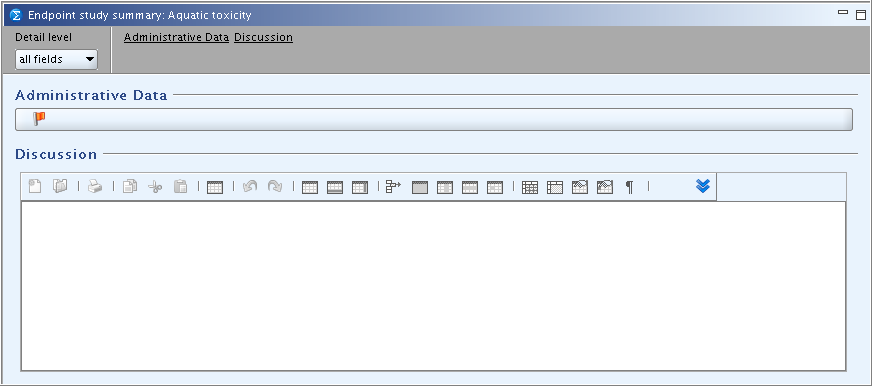
Exceptionally, quite specific templates are provided at the main header level of sections 6 Ecotoxicological Information and 7 Toxicological information (see chapter D.4.8.6.3 Summaries for (eco)toxicological information (PNECs/DNELs)).
To create a new Endpoint summary record
On the section tree, select the desired section where you wish to add an Endpoint summary record.
Right-click the section title and from the menu displayed, click the New Endpoint summary record command.
A new record appears indicated by a blue bullet ![]() . A default record name is generated and displayed
both in the section tree pane and record titlebar. This record name consists
of the section title only. Opposite to default names of Endpoint study records, no consecutive number is
included, because normally only one single Endpoint summary record can be
created per main or subsection. Examples are shown in the following
screenshot:
. A default record name is generated and displayed
both in the section tree pane and record titlebar. This record name consists
of the section title only. Opposite to default names of Endpoint study records, no consecutive number is
included, because normally only one single Endpoint summary record can be
created per main or subsection. Examples are shown in the following
screenshot:
Optionally you can rename records as appropriate, e.g. by adding a specific note. For more information see chapter D.4.8.3 Renaming an Endpoint summary record.
Important
Substance datasets created out of IUCLID 4 datasets by means of the migration tool may contain more than one Endpoint summary record per section, which is necessary in order to match several endpoint summaries in the source dataset. If this is the case, it will not be possible anymore to create an additional new (and empty) Endpoint summary record. Revision of the migrated Endpoint summary records should be considered in such a way that one record is updated, while all others are then deleted.
To rename an Endpoint summary record
Select the record in the section tree pane and either press the F2 key or right-click the record and from the menu displayed, click the Rename command.
Edit the record name as appropriate and then click OK.
For example, change the default name "Melting point/freezing point, IUC4#20/Ch.10.1" (created by the migration tool) to "Melting point/freezing point".
Note
Since only one Endpoint summary record can be created per section, the default name is normally sufficient.
To select and open an Endpoint summary record, you have two options: Either navigate through the section tree until you find the desired summary record; this may be sufficient in most cases because only one Endpoint summary record is normally available per section. Or use the query / filter method, which is the method of choice when you know the record name or part of it.
Option 1: To browse through the section tree and open a record
Click the Plus symbol(s)
 in front of the hierarchical section titles on
the section tree pane in order to expand the subsections as appropriate
until the desired record is displayed.
in front of the hierarchical section titles on
the section tree pane in order to expand the subsections as appropriate
until the desired record is displayed.Either double-click the record or right-click it and from the menu displayed, click the Open command.
Option 2: To query and find a record and open it
Click into the Find or Filter pane
 below the title bar of the section tree
pane.
below the title bar of the section tree
pane.Enter the record name or an adequate part of it. Either double-click the record or right-click it and from the menu displayed, click the Open command.
For example, search string "aqu" will immediately find Endpoint summary record "Aquatic toxicity". See also guidance in chapter D.4.7.4 Selecting and opening an Endpoint Study Record.
To navigate within the data entry screen of an Endpoint summary record you can either use the usual navigation buttons on the keyboard and the scroll bar or, for quick jumps to the main parts, use the navigation bar.
Navigating by keyboard buttons and scroll bar
To navigate through the Endpoint summary record do one of the following:
Use the vertical scroll bar to the right
Use the scroll wheel of your mouse
Press the Page Down or Page Up key on the keyboard
Press the Down Arrow or Up Arrow key on the keyboard
Caution
The use of the navigation keystrokes only works if you are in the Edit mode and if you clicked into a field first. The proper functioning may also depend on your browser. For example, scrolling using the scroll wheel of the mouse may only work if the mouse pointer is somewhere to the right of the data entry screen and not hovering over data entry fields.
Navigating to main parts of an Endpoint summary record
Endpoint summary records are structured into the following main parts:
Administrative data
Short description of key information (except for higher level sections)
Key parameter (optional) (except for higher level sections)
Discussion (or "Results and discussions" in sections 6 Ecotoxicological Information and 7 Toxicological information)
These headings are provided as links in the navigation bar just below the title bar of each Endpoint summary record (see the screenshot below). They offer a quick way to navigate to the main parts of the record, which may be useful if long texts are included in the rich text fields.

In this subchapter general guidance is given on the purpose of the fields provided in Endpoint summary records and the type of information expected to be entered. The guidance given is equivalent to the Online Help which is available on pressing the F1 key or clicking the Help button on the toolbar.
For instructions on the different data types, see chapter D.4.5 What data types are available for input fields and how are they used?.
The following screenshot shows a typical Endpoint summary record related to an individual Endpoint section. General guidance on the data entry fields is provided below.

Administrative data
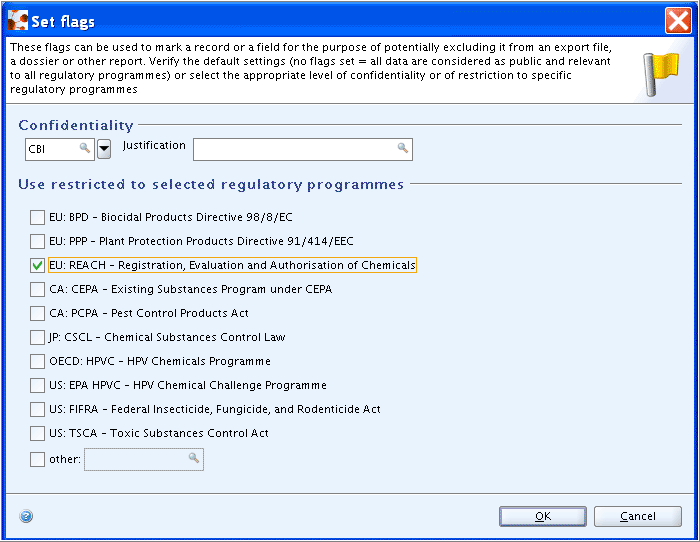
Under this main heading, a flags dialogue box is provided with both
the Confidentiality flag and Regulatory purpose
flags. These can be used to filter out the flagged data in
subsequent operations such as exporting, printing or Dossier
creation.
Note
While it may be useful to flag Endpoint summary records as being relevant only for a specific regulatory purpose, e.g. REACH, setting a confidentiality flag will probably be seldomly used for an endpoint summary. If it is, a justification shall be given in the respective field.
Short description of key information
Under this main heading, a text field is provided for entering a short description of the most relevant endpoint data. This includes in principle the summary information that is compiled e.g. in the OECD Full SIDS Summary format or other endpoint summary formats. Provide only the most relevant details, which could be, depending on the cases, the test guideline used, test organism and exposure duration. Normally no reliability score needs to be indicated as it can be assumed that the studies identified are valid (reliability score 1 or 2). If this is not the case (for instance if the reliability of a published study cannot be assigned or if several less valid studies are used for a weight of evidence analysis), the reliability indicators should be specified.
Also several key studies can be referenced as applicable. However, the characterisation of the endpoint data should be kept as concise as possible. Examples of what could be entered here, depending on the endpoint, are as follows:
Melting point: 54.6-55.8 °C at 1,013 hPa
Water solubility: completely miscible
pH: 6.9 (80 mg/l water) at 20°C (OECD TG105)
Oxidation reduction potential: no data available
Phototransformation in air: T1/2 = 9.32 x 10-2 yr (sensitizer: OH radical) (AOP Win v 1.86)
Biodegradation in water: screening tests: Not readily biodegradable: 0 - 8% (BOD) in 28 days, 0 - 1% (HPLC) in 28 days (OECD TG 301C)
Short-term toxicity to fish:
LC50 (96h) < 100 mg/l for Pimephales promelas (OECD TG 203)
LC50 (48h) = 3.2 mg/l for Oryzias latipes (OECD TG 203)
Acute toxicity:
Dermal: LD50: > 2,000 mg/kg for rat (limit test)
Genetic toxicity:
In vitro:
Gene mutation (Bacterial reverse mutation assay / Ames test): S. typhimurium TA 100: positive with and without metabolic activation; TA 1535: positive without metabolic activation (equivalent to OECD TG 471)
Key parameter (optional)
The field(s) under this heading (if provided) can be optionally completed in order to specify the key parameter(s) that may subsequently be used in the chemical safety assessment, classification and labelling or other. In order to enable the use of any specific software, only a minimum number of structured and hence, searchable fields are provided, in many cases only one.
Key parameters are intended to condense the data summarised in field
Short description of key information to one single
numeric value or concluding remark (e.g. negative / positive) chosen from a
drop-down list. Where a numeric field is provided, only a clear value can be
entered, that is, no range and no less than or greater than qualifiers.
Conversion to a predefined unit as indicated in the field label (e.g. mg/L)
may be required.
If the key value is no clear number, but a range or preceded by <,
<=, >, or >=, you may either leave this field empty or make up a
value you consider most appropriate for the intended subsequent purpose. The
rationale for any user-derived values should be described in field
Discussion for the sake of transparency. Some
non-exhaustive examples of user-derived parameters are as follows:
Aquatic short-term L(E)C50 >100 mg/L (e.g. because only tested up to water solubility of the substance): it may be appropriate to assume 100 mg/L as worst case value.
Aquatic long-term LOEC 1 mg/L (>10 and <20% effect): calculate NOEC as LOEC/2 and enter 0.5 mg/L in the field for NOEC.
Acute oral LD50 >2000 mg/kg b.w. (because no LD50 was achieved in a limit test): enter 2000 mg/kg b.w.
Discussion
In this rich text field, describe the assessment you have made for the given endpoint. Provide the rationale for the choice of the key study(ies) and the choice of the key parameter that, according to your judgment, characterise the endpoint. This includes a discussion of the key information identified and in some instances of studies which are considered to be unreliable, but give critical results. A discussion as to why they were discarded in favour of other studies should then be included. Vice versa, a weight of evidence analysis based on less reliable data or use of published data, the reliability of which cannot be judged because of limited reporting, should be justified.
If several studies were identified to be relevant for the assessment, discuss possible reasons for differing results if any, e.g. differences in purity / impurities of the test substance used, differences in the methods and test conditions, etc.
Justification for classification or non-classification
Provide an appropriate justification for the classification proposed or, when relevant, for the non-classification of the substance. (Note: This field is only be provided for selected sections.)
The following fields are provided with Endpoint summary records for main sections such as 6.1 Aquatic toxicity, under which several Endpoint sections are subsumed:
Administrative data
Under this main heading, a flags dialogue box is provided with both the
Confidentiality flag and Regulatory purpose
flags. These can be used to filter out the flagged data in
subsequent operations such as exporting, printing or Dossier
creation.
Note
While it may be useful to flag Endpoint summary records as being relevant only for a specific regulatory purpose, e.g. REACH, setting a confidentiality flag will probably be seldomly used for an endpoint summary. If it is, a justification shall be given in the respective field.
Discussion
Provide any introductory remarks and/or overall discussion of the endpoints summarised in the subsections subsumed under this section.

At the main header level of sections 6 Ecotoxicological Information and 7 Toxicological information specific templates are provided for recording more integrated information, i.e. PNECs and DNELs, respectively, including relevant input parameters.
Endpoint summary record for 6 Ecotoxicological Information
The following template is provided:
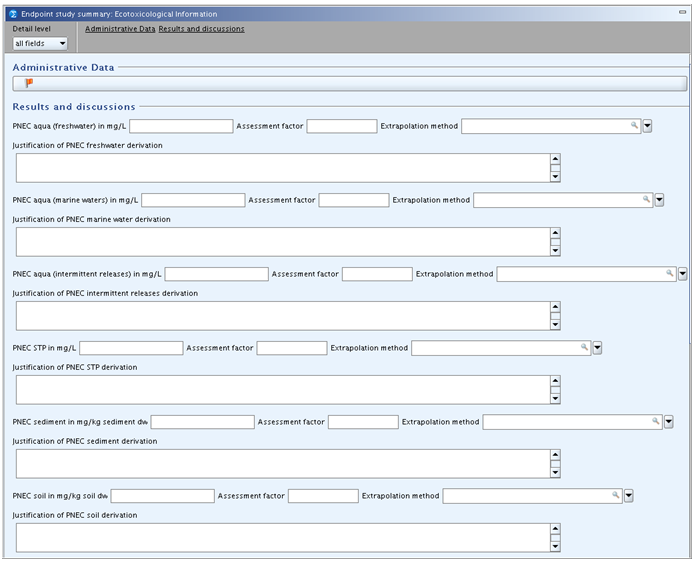
cont'd:
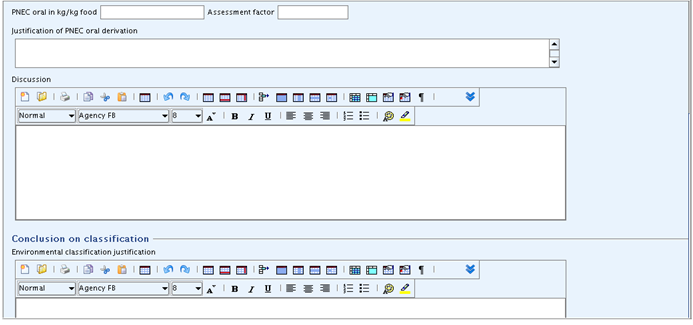
This summary template can be used for recording derived PNEC values (Predicted No Effect Concentrations) including the description of the approach used. Storing the appropriate information here could be of help for the preparation of a CSR, as all justifications for the hazard assessment could be extracted from here. As regards the derivation of these values the relevant guidance document(s) should be consulted, e.g. the Technical Guidance Document (TGD) on preparing the Chemical Safety Report under REACH in its most recent version.
Instructions on how to complete the fields provided in this summary template are as follows:
Administrative data
Under this main heading, a flags dialogue box is provided with both the
Confidentiality flag and Regulatory purpose
flags. These can be used to filter out the flagged data in
subsequent operations such as exporting, printing or Dossier
creation.
Note
While it may be useful to flag this summary record as being relevant only for a specific regulatory purpose, e.g. REACH, setting a confidentiality flag will probably be seldomly used. If it is, a justification shall be given in the respective field.
Results and discussions
Under this heading, the derivation of PNEC values for the environmental compartments (i) aqua (freshwater), (ii) aqua (marine water), (iii) aqua (intermittent release), (iv) STP (sewage treatment plant), (v) sediment, (vi) soil and (vii) oral (food) can be recorded using following fields:
PNEC <compartment> in <unit>: Enter the derived PNEC value in mg/L (aqua and STP), mg/kg sediment dw (sediment), mg/kg soil dw (soil) or kg/kg food (oral), respectively (only numeric value allowed). Convert any value based on other units to the default unit indicated in the field label, when necessary.Assessment factor: Specify the assessment factor used for deriving the PNEC (only whole number allowed), if the assessment factor method was used.Extrapolation method: Specify as appropriate, i.e. select from picklist "assessment factor", "statistical extrapolation" or "partition coefficient" (for sediment and soil only). No such field is provided for compartment "oral (food)" as it is assumed that only an assessment factor method will be used.Justification of PNEC <compartment> derivation: Include a justification of the PNEC value derived. Briefly describe the rationale for the method applied, specify the data which have been used for the derivation of the PNEC, e.g. justify the selection of data amongst the different taxonomic groups (the specific selection of the key studies used for each taxonomic group can be reported in the respective Endpoint summary record) and justify any assessment factors used, e.g. by referring to the standard procedures of the TGD.In the case of PNEC oral, specify the target group to which the PNEC refers (e.g. PNEC oral, predator or PNEC oral, top predator), specify the bioaccumulation potential that triggered the necessary PNEC derivation, and describe the different steps used for the PNEC derivation, including any conversion from NOAELs (toxicity studies) to NOECs and all equations applied.
Discussion: Describe any other relevant criteria as far as not given in the respective fields for the different compartments, and discuss the overall outcome of the PNEC derivations. In this rich text field, you can also insert table(s) either by creating them using the rich text editor or pasting them from a word-processing or html document.
Note
Independent of PNEC values, the field Discussion
can also be used for summarising the environmental hazard assessment
including e.g. discussion of substance-inherent properties that may have
an impact on aquatic as well as soil or sediment organisms.
Conclusion on classification: Environmental classification justification
In this rich text field, you can provide any conclusions drawn towards the environmental classification of the substance including the justification for this classification.
Endpoint summary record for 7 Toxicological Information
The following template is provided:
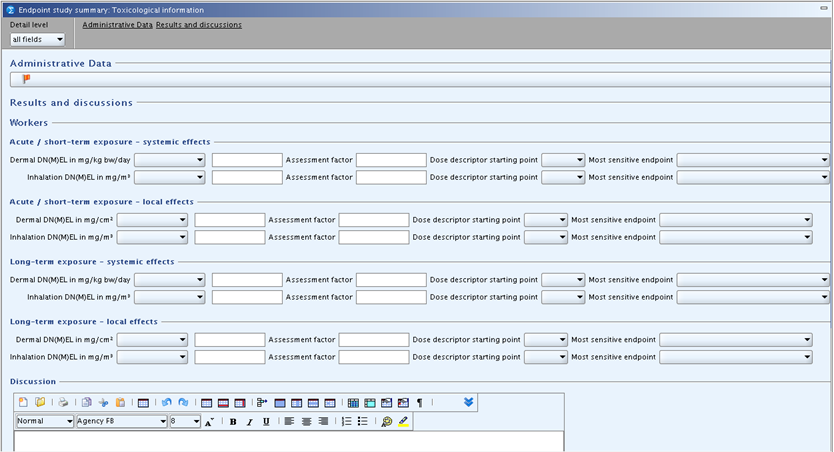
cont'd:
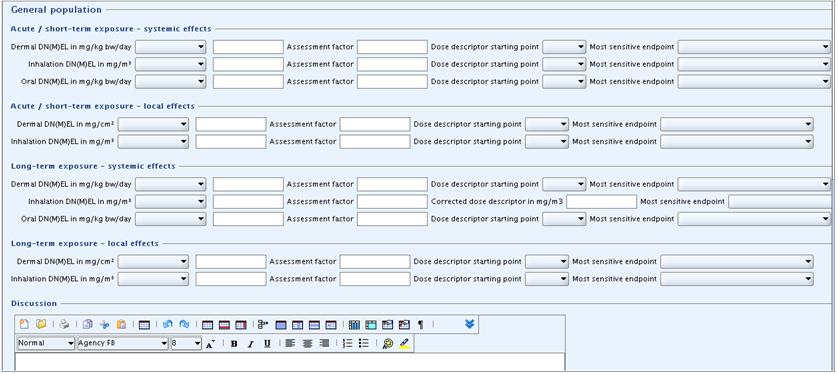
If a human health hazard assessment is required, this summary template should be used for recording information related to the leading health effect for various exposure patterns. The information may be a DNEL (Derived No-Effect Level) value, a DMEL (Derived Minimal Effect Level) value or an indication of "non quantifiable" in case data and/or the effect does not allow quantification. As regards the derivation of these values the relevant guidance document(s) should be consulted, e.g. the Technical Guidance Document (TGD) on preparing the Chemical Safety Report (CSR) under REACH in its most recent version.
Instructions on how to complete the fields provided in this summary template are as follows:
Administrative data
Under this main heading, a flags dialogue box is provided with both the
Confidentiality flag and Regulatory purpose
flags. These can be used to filter out the flagged data in
subsequent operations such as exporting, printing or Dossier
creation.
Note
While it may be useful to flag this summary record as being relevant only for a specific regulatory purpose, e.g. REACH, setting a confidentiality flag will probably be seldomly used. If it is, a justification shall be given in the respective field.
Results and discussions
Under this heading, the derivation of DNEL or DMEL values,
respectively, for systemic or local effects in workers and general
population and for different routes of exposure can be recorded. Please
consult the CSR guidance in relation to which DNELs or DMELs shall be
established. In addition, any exposure scenario(s) may require identifying
such values also for certain vulnerable sub-populations (e.g. children,
pregnant women). No distinct fields are provided in this template for
sensitive sub-populations. Instead, use the field
Discussion for documenting these values, if any.
To record any DN(M)EL values, use the following fields:
Heading WORKERS
Subheading "Acute / short-term exposure - systemic effects":
Under this subheading, DN(M)EL values for systemic effects after acute / short-term dermal or inhalation exposure can be recorded. For definition of acute/short-term in the context of the exposure scenarios please consult the CSR guidance. The following fields are available:
Dermal DN(M)EL in mg/kg bw/day: In the first field, select the derived effect level type, i.e. either DNEL or DMEL or, if applicable, select "not quantifiable". In the related numeric field, enter the corresponding value. Convert any value based on other units to the default unit indicated in the field label, when necessary. Please also consult the CSR guidance in relation to units AND the corresponding time period.Assessment factor: Specify the overall assessment factor used for deriving the DNEL or DMEL, respectively (only whole number allowed). Leave the field blank if "not quantifiable" was selected.Dose descriptor starting point: From the picklist, select the toxicological reference point or dose descriptor of the dose-response relationship used as a basis, i.e. NOAEL, LOAEL, BMDL05, BMD05, BMDL10 or T25. In the case the dose descriptor used is not listed, select "other" and specify it in fieldDiscussion. Leave the field blank if "not quantifiable" was selected. The terms NOAEL, LOAEL or BMD are used to cover concentrations as well as dose levels.Most sensitive endpoint: From the picklist, select the endpoint identified as the critical one for the given exposure pattern. This field should be filled in case a DN(M)EL or if "not quantifiable" was selected.

Subheading "Acute / short-term exposure - local effects":
Under this subheading, DN(M)EL values for local effects after acute / short-term dermal or inhalation exposure can be recorded. The available fields are equivalent to those for systemic effects, except that the unit for dermal DN(M)EL is mg/cm² instead of mg/kg bw/day. Please also consult the CSR guidance in relation to units AND the corresponding time period.
Subheading "Long-term exposure - systemic effects":
Under this subheading, DN(M)EL values for systemic effects after long-term dermal or inhalation exposure can be recorded. The available fields are equivalent to those for acute / short-term systemic effects.
Subheading "Long-term exposure - local effects":
Under this subheading, DN(M)EL values for local effects after long-term dermal or inhalation exposure can be recorded. The available fields are equivalent to those for acute / short-term local effects.
Discussion: Describe any other relevant criteria as far as not given in the distinct fields (e.g. specify the key studies used for the dose descriptors including the target organs identified) and discuss the overall outcome of the DN(M)EL derivations. In this rich text field, you can also insert table(s) either by creating them using the rich text editor or pasting them from a word-processing or html document.
Heading GENERAL POPULATION
Under this heading, DN(M)EL values can be recorded for the same routes and exposure duration types as for workers. In addition, oral DN(M)EL values can be entered for systemic effects, both for acute / short-term and long-term exposure.
Subheadings and fields: same as described above except for following additional fields:
Subheadings "Acute / short-term exposure - systemic effects" and "Long-term exposure - systemic effects":
Oral DN(M)EL in mg/kg bw/day: In the first field, select the derived effect level type, i.e. either DNEL or DMEL or, if applicable, select "not quantifiable". In the related numeric field, enter the corresponding value. Convert any value based on other units to the default unit indicated in the field label, when necessary. Please also consult the CSR guidance in relation to units AND the corresponding time period.Assessment factor: Specify the overall assessment factor used for deriving the DNEL or DMEL, respectively (only whole number allowed). Leave the field blank if "not quantifiable" was selected.Dose descriptor starting point: From the picklist, select the toxicological reference point or dose descriptor of the dose-response relationship used as a basis, i.e. NOAEL, LOAEL, BMDL05, BMD05, BMDL10 or T25. In the case the dose descriptor used is not listed, select "other" and specify it in fieldDiscussion. Leave the field blank if "not quantifiable" was selected. The terms NOAEL, LOAEL or BMD are used to cover concentrations as well as dose levels.Most sensitive endpoint: From the picklist, select the endpoint identified as the critical for the given exposure pattern. This field should be filled in case a DN(M)EL has been selected as well as if "not quantifiable" was selected.
Discussion: Describe any other relevant criteria as far as not given in the distinct fields (e.g. specify the key studies used for the dose descriptors including the target organs identified) and discuss the overall outcome of the DN(M)EL derivations.Important
If DN(M)EL values are available for sensitive sub-populations, document and discuss these values. In this rich text field, you can also insert table(s) either by creating them using the rich text editor or pasting them from a word-processing or html document.
Note
Independent of DN(M)EL values, the field "Discussion" can also be used for summarising endpoint data, which do not fit in any specific Endpoint summary record or include an appraisal of several different endpoints.
Endpoint summary records can be copied and pasted using the clipboard manager as described for the Endpoint study records (see chapter D.4.7.9 Copying Endpoint study records).
References to Endpoint summary records of another Substance dataset can be included using the clipboard manager as described for the Endpoint study records (see chapter D.4.7.10 Referencing Endpoint study records to another Substance dataset).
References to Endpoint summary records of another Substance dataset can be detached as described for the Endpoint study records (see chapter D.4.7.11 Detaching a reference to another Substance dataset).
The Annotations feature, which is available from the Annotations Tab of the Information window, provides a means for making annotations on any record in the Substance dataset including any Endpoint summary record. It is primarily designed for the use by regulatory authorities, e.g. the European Chemicals Agency or Competent Authorities of the Member States, when evaluating the data submitted by the applicant. However, it may also be used by the legal entity compiling a Substance dataset, for example, in the context of the internal review process.
For detailed guidance on how to include annotations refer to chapter D.4.9.6 Tab "Annotations".
Single Endpoint summary records can be printed as described for printing single Endpoint study records (see chapter D.4.7.13 Printing Endpoint study records).
Single Endpoint summary records can be exported as described for exporting single Endpoint study records (see chapter D.4.7.14 Exporting Endpoint study records).
IUCLID provides two options for importing records into a dataset:
Import command for importing records into the clipboard: see chapter D.4.7.9 Copying Endpoint study records.
Import feature available from the Task panel: see chapter D.14 Import (import data from other IUCLID 5 systems).
Single Endpoint summary records can be deleted as described for Endpoint study records (see chapter D.4.7.16 Deleting Endpoint study records).