The creation of a Dossier is very similar to the creation of a print-out with regard to following features:
Flexibility as to which elements shall be included in a Dossier.
A Dossier is created based on a Dossier template (not to be mistaken for the "Template" feature). Dossier templates serve three purposes: (i) They determine the specification of the submission type in the Dossier header displayed in the Navigation window. (ii) They provide data entry fields for administrative information concerning the Dossier. (iii) They predefine the subsections of sections 1 to 3 required, exclude sections that are not relevant for a given Dossier type (e.g. biocides-related sections in a REACH Dossier) and select or deselect specific Endpoint sections by default. In some Dossier templates reflecting Dossier types for which no endpoint data are required, no Endpoint sections are provided at all.
Irrespective of the default selections the user can select or deselect specific records or sections except for some mandatory information in sections 1 to 3.
If documents are related to other IUCLID elements, namely to another Substance, a Category or a Template, all these elements are included in the Document selection dialogues for sections 1 to 3 and sections 4 to 13, respectively, except when Categories are excluded explicitly.
In addition to the Print feature, the Dossier feature provides following possibilities:
If a Substance is part of a Category, it can be decided in a first step already, whether the related category and category member Substances shall be used for Dossier creation or not and, if a Substance is related to several Categories, which of them shall be used.
Additional administrative information concerning the Dossier can be given.
A copy-protect lock can be set, which prevents Endpoint Study / Summary Records provided with a Dossier from being copied.
Tip
It is recommended to select the checkbox Show dossier
colour in the IUCLID5 User Preferences. This will colour the
title bars of Dossiers red and thus, make them more distinguishable from
raw datasets (see chapter D.16.5 Feature "User
Preferences": How to set User Preferences).
Creating a Dossier for a Substance not related to a Category
To create a Dossier for a Substance, follow these steps:
Go Home
 to the Task panel if you are not already
there.
to the Task panel if you are not already
there.Under Substance
 , click Update. A screen comes on with empty windows on
the right side and a Query results pane
on the left (below the title bar Navigation) showing all substances available in
your local IUCLID installation or the network you are connected to. The
Substances are listed in alphabetical order (see below
screenshot).
, click Update. A screen comes on with empty windows on
the right side and a Query results pane
on the left (below the title bar Navigation) showing all substances available in
your local IUCLID installation or the network you are connected to. The
Substances are listed in alphabetical order (see below
screenshot).On the Query results pane, right-click the desired Substance and from the menu displayed, click the Create dossier command (If there is a large number of Substances listed, run a query as described in chapter D.4.3.2 Querying for a Substance on the Query results pane.)
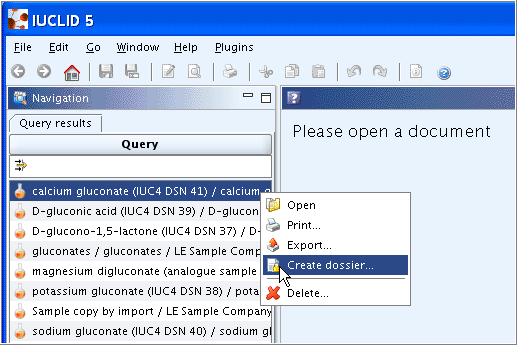
The Create dossier wizard comes up and guides you through a several steps dialogue: Verify or change the default properties (i.e. for which the records shall be included in the dossier), specify specific dossier information, and click the Finish button.
Tip
Note the tips given for Step 5 of the Create dossier wizard as to how several Endpoint records or complete sections can be quickly selected or deselected.
The following screenshots illustrate the dialogues of the Create Dossier wizard:
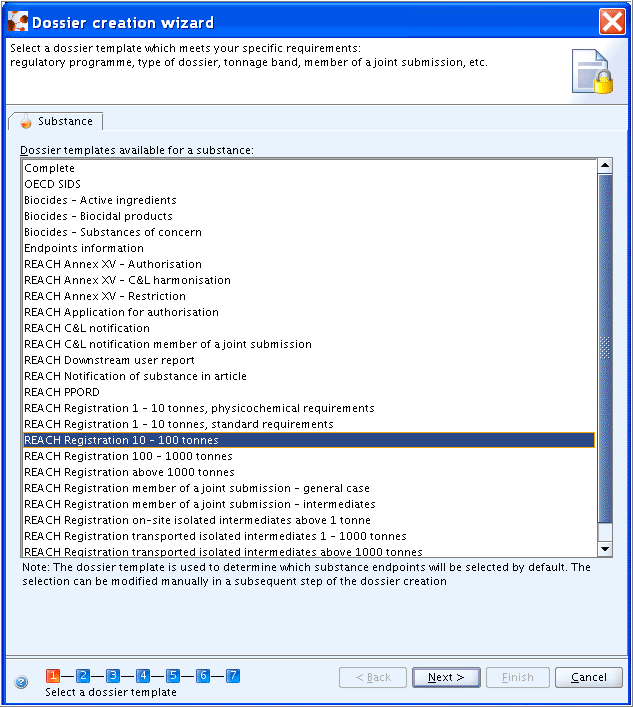
Step 1: Unless you wish to include all sections (i.e. type "Complete"), select the Dossier template type that shall be used, as shown in the following screenshot.
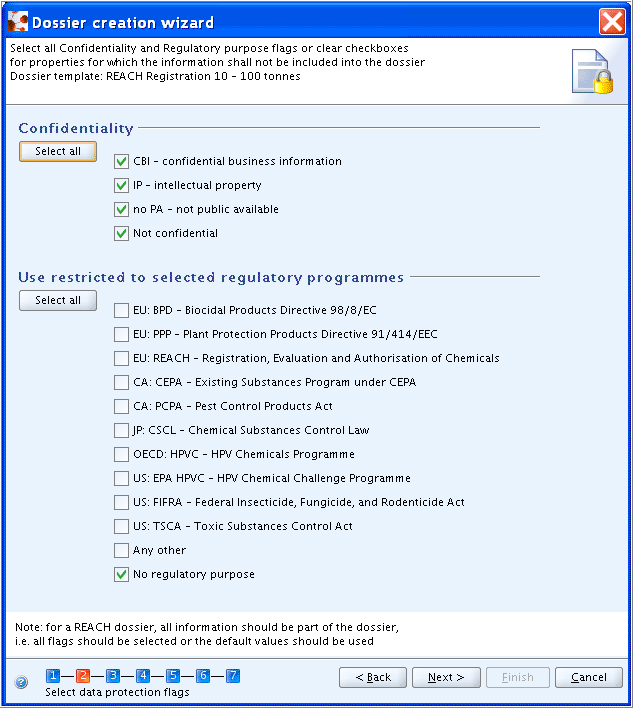
Step 2: Click both Select all buttons to select all Confidentiality and Regulatory purpose flags or clear the checkboxes for properties for which records shall not be included in the Dossier.
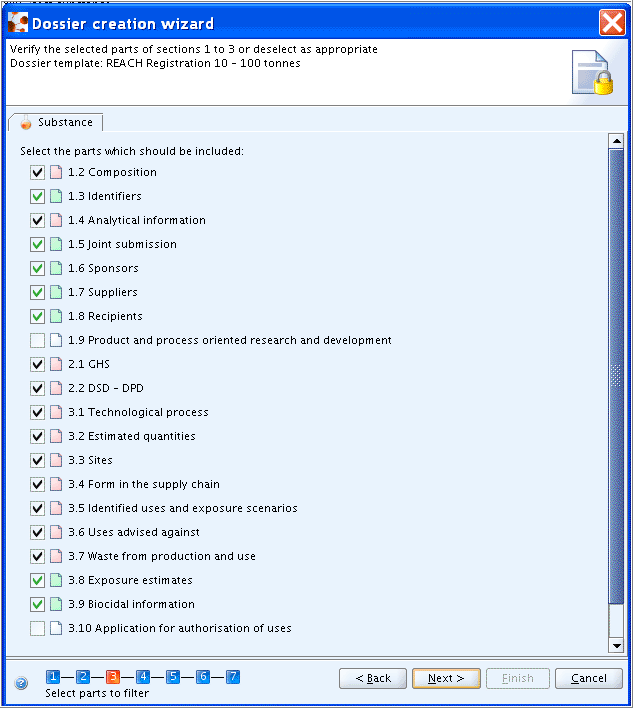
Step 3: Click the Next button if the default settings apply (i.e. all selected subsections of sections 1 to 3) or select/deselect as appropriate. Note that checkboxes with black ticks cannot be deselected.
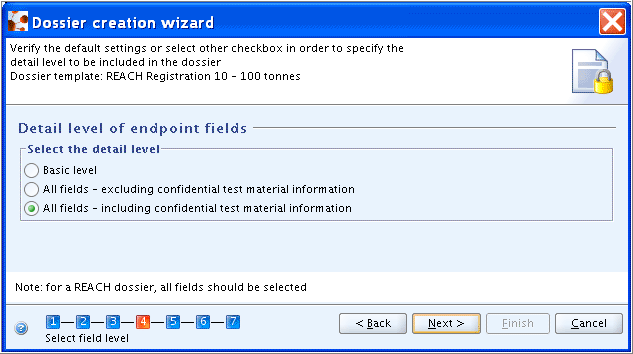
Step 4: Click the Next button if default settings apply (i.e. "All fields - including confidential test material information" selected) or select other checkbox in order to specify the detail level to be included in the Dossier.
Step 5: Verify the selected Endpoint study / summary records or deselect as appropriate. Click the Next button to confirm.
Note
It is not possible to deselect the Substance, Template, Category, Legal entity or Reference substance records although indicated so in the help text of the dialogue box.
Tip
Be aware of the following features:
Sort the selection table alphabetically by Endpoint section: Click into the column title "Endpoint". Repeated clicking changes the sort direction.
Use the appropriate Select / Deselect commands available by right-clicking into the table, i.e.:
Current: Selects or deselects the currently marked records. For example, mark all records of a section by clicking the first record, hold down the Shift key and click the last record of that section. Then right-click into the marked area and select the Current command for Select or Deselect as appropriate.
All: Selects or deselects all records except the ones checked with a black tick.
By type: Selects or deselects all records of either of the types Attachment or Endpoint study record.
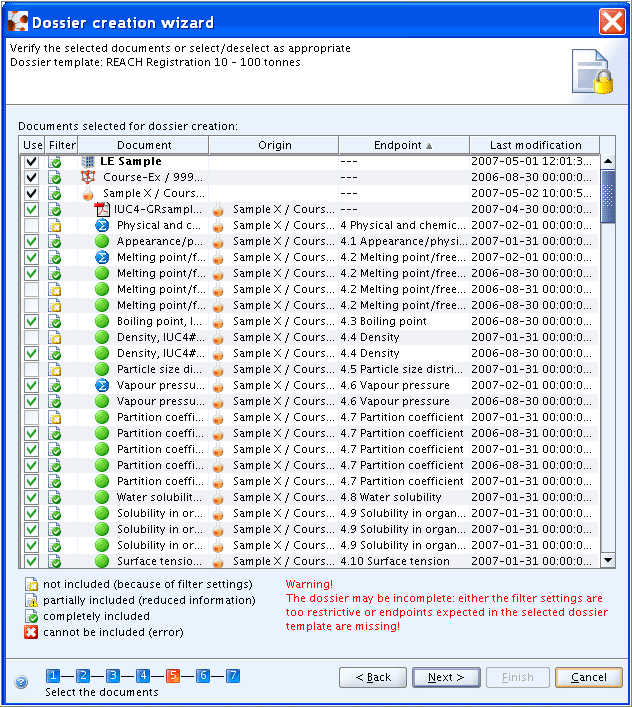
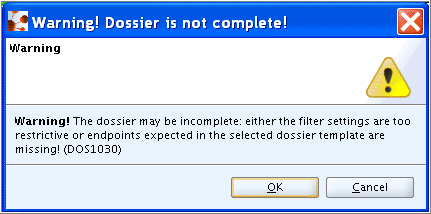
The following warning may come up, when not all sections required for the selected Dossier template are filled with endpoint data (independent of whether the available data fulfill the requirements or not):
Step 6: Enter administrative data as prompted by the self-explanatory fields. Different types of templates for administrative fields are provided depending on the Dossier template selected (see details in chapters D.8.2.1 Creating Dossiers for different REACH requirements, D.8.2.2 Creating Dossiers for Biocides, D.8.2.3 Creating a Dossier for OECD HPVC.). In addition, a rather generic template, prompting for a
NameandDossier submission remarkonly, is provided for Dossier types "Complete" and "Endpoint information", which are not related to any specific regulatory programme. The following screenshot example shows one of two types used for REACH Dossiers: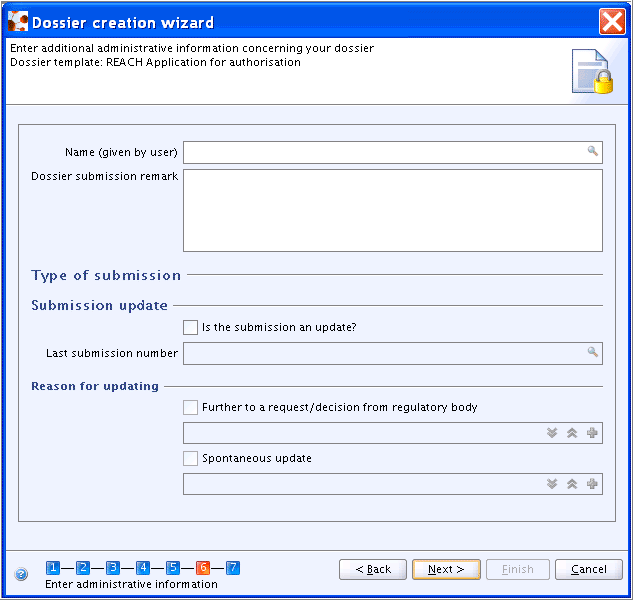
Step 7: Select the Copy-protect checkbox, if the ownership of the Dossier data shall be protected in such a way that copying of Endpoint Study / Summary Records using the IUCLID clipboard manager will be prevented and click the Finish button.
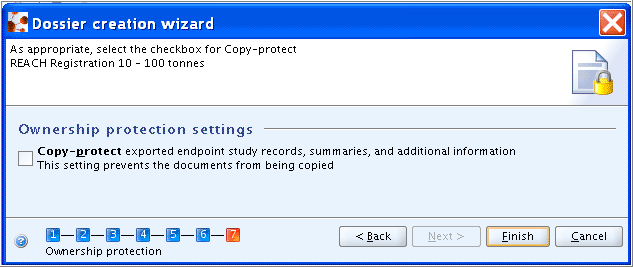
Creating a Dossier for a Substance that is related to Category(ies)
Only if a Substance is related to one or more Categories, the first step consists of a dialogue box, in which you can specify whether the related Category shall be included in the Dossier or not (see also chapter D.8.2.5 Creating a Dossier for a Category). When the radio button Select category(ies) is selected, a drop-down list occurs from which the respective Category can be selected, as shown in following screenshot:.
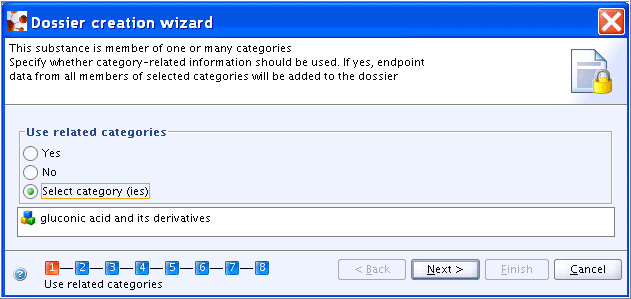
All other steps are the same as steps 1 to 7 for Creating a Dossier for a Substance not related to a Category.
For guidance on different options preferred in the context of OECD HPVC and REACH, see chapter D.8.2.5 Creating a Dossier for a Category.
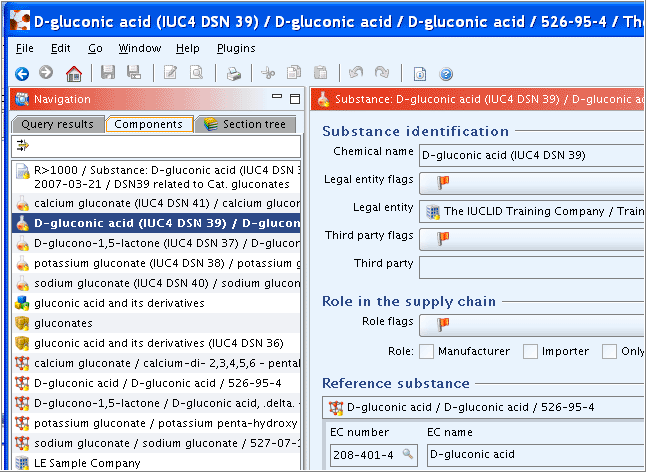
Dossier components displayed in the Components pane
The Dossier created appears in a new tab of the Navigation window, called Components. Each component of a Dossier is listed, i.e.:
The Dossier itself:
The Dossier title includes the Dossier template type, the name of the Substance / Reference substance, the CAS No., Legal entity, date and the user-defined name.
The Dossier information entered during the process of Dossier creation is displayed (read-only) on the Data entry window. You can directly navigate to the Dossier subject. See below screenshot.
The Dossier-related Information window.
The source Substance dataset
The Reference substance referred to in the Substance
The Legal entity the Substance is assigned to
The following screenshot shows an example of Dossier components and part of the Dossier information:
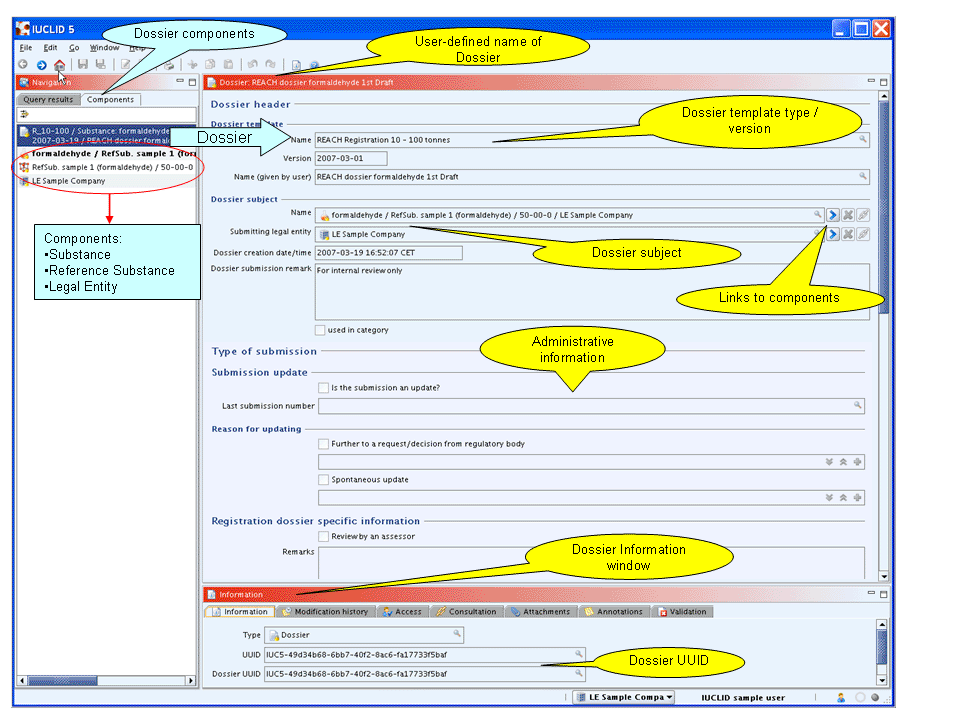
How to navigate to the Dossier components and review Dossier data
As described above, all Dossier components are listed in the Components tab. The following components can appear depending on the source documents included in a Dossier:
 Dossier: This includes the actual Dossier
description as displayed in the right screen.
Dossier: This includes the actual Dossier
description as displayed in the right screen. Substance: Link(s) to the Substance dataset(s)
included in the Dossier.
Substance: Link(s) to the Substance dataset(s)
included in the Dossier. Template: Link(s) to the Template(s) assigned to
the Substance.
Template: Link(s) to the Template(s) assigned to
the Substance. Category: Link to the Category to which the
Substance included in the Dossier are assigned ( for more information
see chapter D.8.2.5 Creating a Dossier for a
Category).
Category: Link to the Category to which the
Substance included in the Dossier are assigned ( for more information
see chapter D.8.2.5 Creating a Dossier for a
Category). Mixture: Link to the Mixture to which the
Substances included in the Dossier are assigned ( for more information
see chapter D.8.2.4 Creating a Dossier for
Mixture).
Mixture: Link to the Mixture to which the
Substances included in the Dossier are assigned ( for more information
see chapter D.8.2.4 Creating a Dossier for
Mixture). Reference substance: Link(s) to the Reference
Substance(s) assigned to the Substance(s).
Reference substance: Link(s) to the Reference
Substance(s) assigned to the Substance(s). Legal entity: Link(s) to the Legal entity(s)
assigned to the Substance(s).
Legal entity: Link(s) to the Legal entity(s)
assigned to the Substance(s). Legal entity site: Link(s) to the Legal entity
Site(s) assigned to the Substance(s).
Legal entity site: Link(s) to the Legal entity
Site(s) assigned to the Substance(s).
Important
All components provided with a Dossier can be viewed, but not
edited. The underlying raw data are "frozen" in the Dossier. This is also
optically indicated by a yellow Lock symbol attached to any of the
component symbols as in the Dossier symbol: ![]() .
.
You can navigate to each of the components by double-clicking it in
the Components pane. The Dossier subject, i.e. the Substance, Category or
Mixture, and the submitting Legal entity can be also be accessed by clicking
the respective link buttons ![]() in the Dossier part "Dossier subject" (see above
screenshot).
in the Dossier part "Dossier subject" (see above
screenshot).
Navigating to a component means that it is opened in the read-only mode and all contained elements, e.g. Endpoint study / summary records, can be accessed. Any links available within the Dossier components remain active. For example, you can access the category member Substances from within the Category component. If the Dossier component you open is any element that contains Endpoint sections, i.e. a Substance, Template or Mixture, the Navigation pane is further subdivided and a third pane, i.e. the Section tree pane, occurs (see the following screenshot). You can use this pane to navigate to Endpoint sections as you would when editing a Substance (see chapter D.4.3.3.1 User interface of Substance dataset: structure and elements).
How to print individual Dossier components
As further described in chapter D.8.7 Printing a Dossier, not only a complete Dossier, but also any individual Dossier component can be printed to an HTML file. This may be useful e.g. for the internal review process or in other cases where the number of redundant data should be limited to a minimum.
Caution
The Print command, available from the File menu, does not print the Dossier component highlighted on the Component pane, but the one opened in the right (large) window. Make sure first to double-click the desired component or right-click it and from the menu displayed, click the Open command. Then start the Print command.
In step 1 of the Dossier creation wizard (see chapter D.8.2 Creating Dossiers), a dialogue box is provided in which you can select the appropriate Dossier template. The administrative fields provided in step 6 appear in the Dossier. The available REACH Dossier templates are based on the following two types of administrative fields, which are denoted as REACH 1 and REACH 2 in the lists below:
REACH 1:

REACH 2:
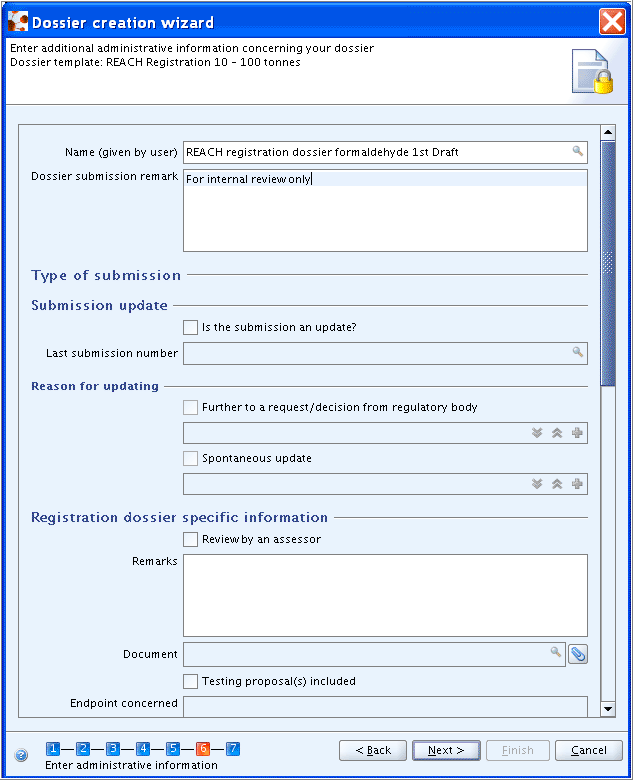
REACH 2 (cont'd):
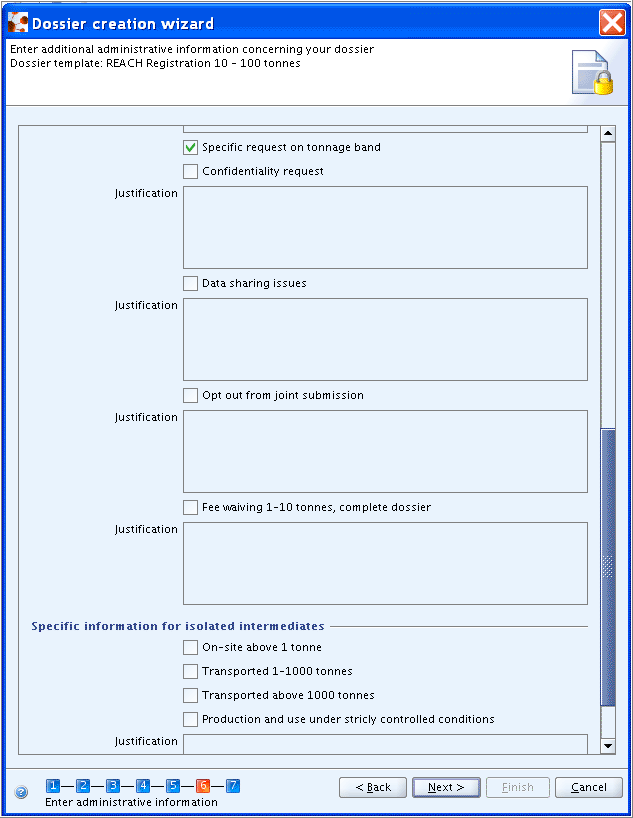
Note
Specific information requested for registration dossiers concerns the following REACH data requirements:
Review by an assessor: REACH, Art. 10 (a)(viii). The possibility is given to add a justification and/or to insert the assessment document.Testing proposal(s) included: REACH, Art. 10 (a) (ix).Confidentiality request: REACH, Art. 10 (a) (xi). A separate fieldSpecific request on tonnage bandis provided to claim confidentiality on the tonnage band.Data sharing issues: REACH Art. 30 (3).Opt out from Joint submission: REACH, Art. 11 (3), Art. 19 (2).
Fee waiving 1-10 tonnes, complete dossier: REACH, Art. 74 (2).
For isolated intermediates (on-site or transported), refer to the regulatory programme guidance to identify whether a separate dossier is required. Specific information on the type of intermediates should be entered in the following fields:
On-site above 1 tonnes.Transported 1-1000 tonnes.Transported above 1000 tonnes.Production and use under strictly controlled conditions: REACH, Art. 17 (3), Art. 18 (4).
In the following, all available Dossier templates are listed along with the type of administrative fields (i.e. REACH 1 or REACH 2) and the default settings with regard to available and selected sections:
Dossiers or reports related to specific submissions by industry
REACH C&L notification: REACH 1; all available Endpoint records deselected by default; sections 9, 10, 11 and 13 excluded. Endpoint records can be added to the Dossier e.g. when it is necessary to justify a classification by providing the Endpoint study record.
REACH C&L notification member of a joint submission: REACH 1; all available Endpoint records deselected by default; sections 9, 10, 11 and 13 excluded (see also chapter D.8.2.6 Creating a joint submission Dossier).
REACH Notification of substance in article: REACH 1; sections 4 to 13 excluded.
REACH Downstream user report: REACH 1; all available Endpoint records deselected by default; sections 9, 10 and 11 excluded. Endpoint records can be added to the Dossier e.g. if a Downstream user reports the results of a study for which he had initially submitted a testing proposal.
REACH PPORD (Product and process orientated research and development): REACH 1; all available Endpoint records deselected by default; sections 9 and 10 excluded. Endpoint records can be added to the Dossier, e.g. to justify by the results of study the exemption for general registration.
REACH Registration 1 - 10 tonnes, physicochemical requirements: REACH 2; all available Endpoint records selected by default; sections 9 and 10 excluded.
REACH Registration 1 - 10 tonnes, standard requirements: REACH 2; all available Endpoint records selected by default; sections 9 and 10 excluded.
REACH Registration 10 - 100 tonnes: REACH 2; all available Endpoint records selected by default; sections 9 and 10 excluded.
REACH Registration 100 - 1000 tonnes: REACH 2; all available Endpoint records selected by default; sections 9 and 10 excluded.
REACH Registration above 1000 tonnes: REACH 2; all available Endpoint records selected by default; sections 9 and 10 excluded.
REACH Registration member of a joint submission - general case: REACH 2; all available Endpoint records deselected by default; sections 11 and 13 selected by default; sections 9 and 10 excluded (see also chapter D.8.2.6 Creating a joint submission Dossier). Endpoint records can be added to the Dossier, e.g. in case of opt-out.
REACH Registration member of a joint submission - intermediates: REACH 2; all available Endpoint records deselected by default; section 4 (Endpoint summary only) and sections 11 and 13 selected by default; sections 9 and 10 excluded (see also chapter D.8.2.6 Creating a joint submission Dossier). Endpoint records can be added to the Dossier, e.g. in case of opt-out.
REACH Registration on-site isolated intermediates above 1 tonne: REACH 2; all available Endpoint records selected by default; sections 9 and 10 excluded.
REACH Registration transported isolated intermediates 1 - 1000 tonnes: REACH 2; all available Endpoint records selected by default; sections 9 and 10 excluded.
REACH Registration transported isolated intermediates above 1000 tonnes: REACH 2; all available Endpoint records selected by default; sections 9 and 10 excluded.
REACH Application for authorisation: REACH 1; section 13 selected by default; sections 4 to 12 excluded.
Dossiers generated by the European Chemicals Agency or Competent Authorities of the Member States
REACH Substance Evaluation: REACH 1; all available Endpoint records selected by default; sections 9 and 10 excluded.
REACH Annex XV - Authorisation: REACH 1; all available Endpoint records selected by default; sections 9 and 10 excluded.
REACH Annex XV - C&L harmonisation: REACH 1; all available Endpoint records selected by default; sections 9 and 10 excluded.
REACH Annex XV - Restriction: REACH 1; all available Endpoint records selected by default; sections 9 and 10 excluded.
Note
In some of the Dossier templates listed above all available Endpoint records are selected by default. This is because the REACH regulation makes it clear that all information should be considered. That is, not only the information requirements referred to in Article 10, but all other available and relevant information on the substance regardless whether testing for a given endpoint is required or not at the specific tonnage level. Irrespective of this the user has the possibility to deselect any records as appropriate.
In step 1 of the Dossier creation wizard (see chapter D.8.2 Creating Dossiers), a dialogue box is provided in which you can select the appropriate Dossier template. The three biocides templates listed below are all based on the same template type. The following templates tailored to the requirements laid down in the EU BPD are available:
Biocides - Active ingredients: All available Endpoint records selected by default.
Biocides - Biocidal products: All available Endpoint records selected by default.
Biocides - Substances of concern: All available Endpoint records selected by default.
The administrative fields provided in step 6 of the Dossier creation wizard are also identical. An example is shown in the following screenshot:
Important
For biocidal products comprising intentional mixtures (i.e. preparations), the IUCLID element Mixture must be used instead of Substance along with the template type "Biocides - Biocidal products". See guidance in chapters D.8.2.4 Creating a Dossier for a Mixture and D.7 Mixture (create and update mixture related information). If this template type is erroneously selected for a Substance, an error message "Please select a substance template!" occurs in the dialogue box.
In step 1 of the Dossier creation wizard (see chapter D.8.2 Creating Dossiers), a dialogue box is provided in which you can select the appropriate Dossier template. There is one OECD SIDS template available:
OECD SIDS: all available Endpoint records selected by default; sections 8, 9, 10 and 11 excluded.
The following administrative fields are provided in step 6 of the Dossier creation wizard:
Creating a Dossier for a Mixture follows the same procedure as described for the Substance (see chapter D.8.2 Creating Dossiers). The only difference is that such a Dossier will contain a Mixture dataset instead of a Substance dataset as primary component, plus all related individual Substance datasets as additional components (see also the components listed under How to navigate to the Dossier components and review Dossier data.)
In a first step of the Dossier creation wizard, the user can decide whether all related Substances shall be included in the Dossier, as shown in the following screenshot:
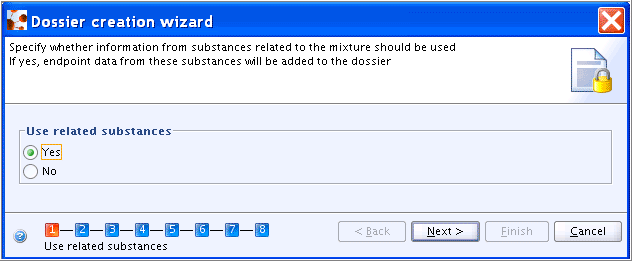
Currently two different approaches are used for creating Dossiers for Categories and Substances related to Categories, i.e.:
OECD approach
REACH approach
The major difference between these two approaches is that OECD does not require to create Dossiers for each individual category member Substance in addition to the Category Dossier, while in the context of REACH a Dossier has to be created for each Substance including the Category information and the Endpoint records of the other category members.
A Dossier is created for the Category as a starting point, which is therefore the Category subject. It includes all assigned category member Substances, related Reference substances, Legal entities, Legal entity sites and any related Templates.
Creating a Dossier for a Category follows the same procedure as described for the Substance (see chapter D.8.2 Creating Dossiers). The only difference is that such a Dossier will contain the Category as primary component. To create a Dossier for a Category, follow these steps:
Go Home
 to the Task panel if you are not already
there.
to the Task panel if you are not already
there.Under Category
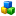 , click Update. A screen comes up with empty windows on
the right side and a Query results pane
on the left (below the title bar Navigation) showing all Categories available in
your local IUCLID installation or the network you are connected
to.
, click Update. A screen comes up with empty windows on
the right side and a Query results pane
on the left (below the title bar Navigation) showing all Categories available in
your local IUCLID installation or the network you are connected
to.In the Query results pane, right-click the desired Category and from the menu displayed, click the Create dossier command (If there is a large number of Substances listed, run a query as described in chapter D.4.3.2 Querying for a Substance in the Query results pane.)
The Create dossier wizard comes up and guides you through a several steps dialogue, which are equivalent to those described in chapter D.8.2 Creating Dossiers: Verify or change the default properties (i.e. for which the records shall be included in the dossier), specify specific dossier information, and click the Finish button.
The following screenshot shows how the Dossier of a Category is displayed in the Components pane. Note that the Dossier subject, i.e. the Category, is highlighted (set in bold).
REACH require creating Dossiers for each individual category member Substance including all relations to other Substances and Categories. Starting point and hence, Dossier subject is an individual Substance. However, all related documents will be included in the Dossier, i.e. all Categories referring to the selected Substance with their other associated Substances. For example, if Substances #1, #2 and #3 belong to Category A and Substances #3, #4 and #5 are part of Category B, a Dossier for Substance #1 will include:
Substance #1 as Dossier subject
Category A
Category B
Substance #2: only Endpoint records and section 1.2 Composition
Substance #3: only Endpoint records and section 1.2 Composition
All Reference substances, Legal entities, Legal entity sites (except for the other category members) and Templates related to each of above elements.
Other Categories referencing Substance #4 or #5 would not be included in the Dossier.
Note
Adhere to the relevant technical guidance documents provided for REACH as to what documents shall be included in Dossiers of Substances that belong to a Category or to Categories. The Create Dossier feature is flexible enough to define any special use cases. In principle, for each Dossier component any endpoint data can be excluded from the Dossier, e.g. either complete sections or selected Endpoint study / summary records. Non-Endpoint sections 1 to 3 (except for section 1.2 Composition) are excluded for other category member Substances.
Tip
Particularly if a Dossier is used for the internal review process before the final version is submitted to the responsible Agency or Competent Authorities, it may be useful to limit the number of redundant data to a minimum. This can be achieved as follows:
Create a Dossier for the Substance only, i.e. without related Categories.
If you create a Dossier for the Substance including related Categories, you can still print each individual component to an HTML (see chapter D.8.7 Printing a Dossier).
To create a Dossier for a Substance that is part of a Category or several Categories, follow these steps:
Go Home
 to the Task panel if you are not already
there.
to the Task panel if you are not already
there.Under Substance
 , click Update. A screen comes up with empty windows on
the right side and a Query results pane
on the left (below the title bar Navigation) showing all substances available in
your local IUCLID installation or the network you are connected to. The
Substances are listed in alphabetical order (see below
screenshot).
, click Update. A screen comes up with empty windows on
the right side and a Query results pane
on the left (below the title bar Navigation) showing all substances available in
your local IUCLID installation or the network you are connected to. The
Substances are listed in alphabetical order (see below
screenshot).In the Query results pane, right-click the desired Substance and from the menu displayed, click the Create dossier command (If there is a large number of Substances listed, run a query as described in chapter D.4.3.2 Querying for a Substance in the Query results pane.)

The Create dossier wizard comes up and guides you through a several steps dialogue, which are equivalent to those described for Creating a Dossier for a Substance not related to a Category, except that the use of related Categories can be specified in a first step, as shown in following screenshot:

Specify whether the related Categories shall be included in the Dossier or not. If "Yes" is selected, all related Categories and Substances assigned to them are included in the Dossier. If the radio button Select categor(ies) is selected, a drop-down list occurs from which the respective Category can be chosen, as shown in above screenshot.
All other steps are the same as steps 1 to 7 for Creating a Dossier for a Substance not related to a Category.
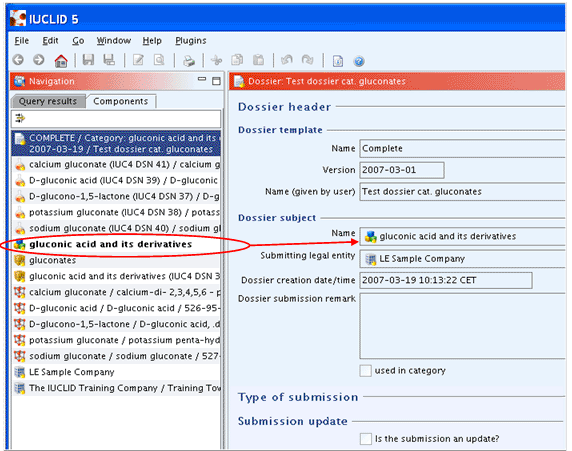
The following screenshot shows how the Dossier of a Substance, which is related to Category(ies), is displayed in the Components pane. Note that the Dossier subject, i.e. the Substance for which the Dossier was created, is highlighted (set in bold).
In the case of multiple registrants for the same substance, the joint submission of a Dossier may be desired or required. A joint submission, sometimes also called as "One substance, one registration" (OSOR) approach, can increase the efficiency of the registration system, reduce costs and reduce testing on vertebrate animals.
Specific provisions may be taken into account when this approach is used, as laid down in Article 11 of REACH. According to these rules, the joint submission consists of sharing technical data and in particular information related to the intrinsic properties of the substance, while the exchanging of information concerning the production capacities, production or sales volumes, import volumes or market shares is not foreseen. A registrant may even claim to submit the information related to the intrinsic properties of the substance separately, if an appropriate explanation is submitted along with the dossier.
A joint submission of data can be managed using IUCLID based on the available features for handling Substance/Mixture datasets, Categories and Dossiers. As outlined in chapter E.1.5 Joint submission, the lead Legal entity has to create the joint submission in his local IUCLID installation and specify the Legal entities of all other participants (named "members of the joint submission" in IUCLID terminology). The lead Legal entity manages the data which are intended to be included in the joint submission, while the members manage the data they wish to submit separately. In the end, a joint submission Dossier will be submitted by the lead Legal entity. Subsequently, each registrant has to submit separately the information that does not have to be included in the joint submission.
In the following, the specific workflows to be taken into account are described.
Workflow of a joint submission for REACH
The lead Legal entity creates the appropriate IUCLID element(s) and specifies the relevant joint submission indicators, i.e.:
In a Substance dataset, section 1.5 Joint submission shall be completed including the identification of the member Legal entities (see chapter E.1.5 Joint submission).
In a Mixture dataset and its related Substance datasets, section 1.5 Joint submission shall be completed including the identification of the member Legal entities (see chapter E.1.5 Joint submission).
In a Category, no specific field for indicating a joint submission is available. It is recommended to include the respective information in field
Remarksunder the heading "General information" (see chapter D.6.2.3.1 General Category information). For all assigned category member datasets, section 1.5 Joint submission shall be completed.For any information that needs to be flagged "confidential", the correct confidentiality flag shall be selected, i.e. flag "IP (Intellectual property", which means that the data should only be provided to other companies when they are trusted (e.g. consortia or with letter of access); the data must not be disseminated to the public (for details on the confidentiality flag, see chapter D.4.7.7.1 Administrative data).
The lead Legal entity and the member Legal entities may contribute to their joint submission by different means depending on how the consortium defines the rules for exchanging data during the process of data compilation, e.g.:
Data can be submitted to the lead through export files.
Data can be exchanged for review based on exported raw data, which can either be
open to edit or
copy-protected or
sealed, i.e. preventing the document from being modified.
Data can be exchanged for review based on print-outs or write-protected Dossiers.
The Annotations feature can be used within the consortium to add comments on any information within a IUCLID element (see chapter D.4.9.6.2 How to manage annotation records).
Once the data for the joint submission are complete and agreed by all registrants, a joint submission Dossier can be created by the lead Legal entity using the respective feature described in chapter D.8.2 Creating Dossiers.
Workflow of separate submissions by registrants other than the lead
Each Legal entity participating in the joint submission creates the IUCLID element(s) needed to store the data that are intended to be submitted separately, i.e. the identity of the manufacturer or importer, information on the manufacture and use(s) and exposure information (for substances in quantities of 1 to 10 tonnes) and, optionally, guidance on safe use and the attached chemical safety report (if required).
The following recommendations are given for these separate submissions:
View mode selector: When you prepare a Substance or Mixture dataset for the information in question, select the view mode tailored to the intended submission, e.g. "REACH Registration member of a joint submission - general case". The respective data requirements (i.e. IUCLID sections) are then indicated in red as described in chapter D.4.3.3.1 Section tree pane.
Section 1.5 Joint submission: Complete this section when you prepare a Substance or Mixture dataset. Identify Legal entities of the lead and all registrants intending the joint submission. In addition, a remark should be entered in field
Remarksindicating e.g. "Separate submission of the information specified in Article 10(a)(i), (ii), (iii) and (x), and any relevant indication under Article 10(a)(viii)".Category: The Category itself will be submitted by the lead Legal entity. Only information on the assigned substance(s) can be submitted separately by the member Legal entities. These can be handled as any other Substance dataset.
Justification for separate submission of specific information: If a registrant submits any information separately that would normally be included in the joint submission, an appropriate explanation has to be submitted along with the Dossier. This can be done by including a short note in field
Remarksof section 1.5 Joint submission referring to an appropriate document attached, in which it is explained as to why the costs would be disproportionate, why disclosure of information was likely to lead to substantial commercial detriment or the nature of the disagreement, as the case may be.For any information that needs to be flagged "confidential", the correct confidentiality flag shall be selected, i.e. flag "CBI (Confidential business information)" which means that the data must not be provided to other companies or disseminated to the public (for details on the confidentiality flag, see chapter D.4.7.7.1 Administrative data).
Once the lead has made the joint submission, each other Legal entity can create and submit a Dossier for the confidential data using the respective feature described in chapter D.8.2 Creating Dossiers.
Tip
The following hints should be taken into account:
It is recommended that the lead submits the joint submission Dossier first.
If a Legal entity participating in the joint submission needs to include any specific (confidential) endpoint data in the Dossier, the respective Endpoint records can be selected in the selection table appearing in step 6 of the Dossier creation wizard (see chapter D.8.2 Creating Dossiers).
If data stored in a Substance or Mixture dataset deemed to be used in the context of a joint submission should also be used for other purposes (e.g. for a separate submission for another regulatory programme), an adequate selection can be made of sections and/or Endpoint records to be included in that Dossier. For instance, section 1.5 Joint submission can be excluded, while other sections are included.