Substance-related information is primarily managed based in Substance datasets. A substance can be defined in different ways; it can be seen as a chemical element, a molecule, or a combination of molecules derived from any manufacturing process or from a biological source. Depending on the regulatory programme considered, the notion of substance may vary. For instance, the REACH regulation defines the substance as a "chemical element and its compounds in the natural state or obtained by any manufacturing process, including any additive necessary to preserve its stability and any impurity deriving from the process used, but excluding any solvent which may be separated without affecting the stability of the substance or changing its composition."
All substance-related information is managed in the Substance dataset. This is the central core of information in IUCLID. It contains all data related to a chemical substance like the chemical identity including the substance composition, information on manufacture, use and exposure, information on the classification and labelling, and all required and available endpoint study summaries. A Substance dataset is the repository of data, which is used to create a Dossier for the substance to be registered. See chapter D.4 Substance (Create and update substance-related information).
Some information required by regulatory programmes, especially REACH, is not directly stored in the Substance dataset. It includes:
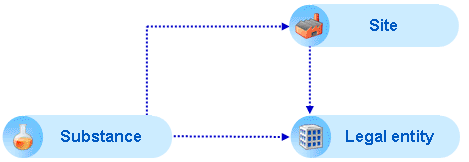
Legal entity: As pointed out in chapter B.4.1 Company-/organisation-related information, all Company-/organisation-related information is maintained in this specific IUCLID element. In the Substance dataset, the company or organisation is identified by means of a link to the Legal entity. Any revisions of the Legal entity will automatically apply to all associated datasets. A Substance dataset is always associated to an owner (Legal entity) and it is not possible to create a dataset without this link. For more information, see chapter D.9 Legal entity (Create and update company-/organisation-related information).

Legal entity site: The Legal entity is associated with a Legal entity site, which in turn is the physical location where the substance is produced and/or used. For more information, see chapter D.10 Legal entity sites (Create and update Legal entity sites).
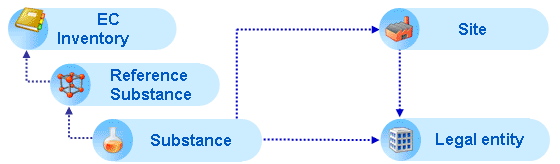
Reference substance: When a Substance dataset is created for a given chemical substance, the identity of the substance is specified by linking the dataset to the appropriate Reference substance, which in turn is based on the EC Inventory or, if not listed, newly defined. For more information, see chapter D.11 Reference substance (Create and update Reference substance related information).
A Substance dataset is structured into 13 main sections (see D.4.3.4.1 What is a IUCLID section?). Many of these sections are further broken down to subsections. These sections contain two very distinct types of information, as further described in the following subchapters, i.e.:
General (non-endpoint) information
Endpoint-related information
Note
IUCLID also provides a Mixture dataset type, which is to be used for preparations only, e.g. for biocidal products (The term "mixture" comes from the terminology used in the Globally Harmonised System of classification and labelling of chemicals (GHS). Mixture datasets are similar to Substance datasets, although it is not the identity of the mixture (i.e. preparation) which is linked to a Reference substance, but the identity of its components detailed in section 1.2 Composition. For more information, see chapter D.7 Mixture (Create and update mixture related information). Be aware that unintentional mixtures consisting of more than one constituent as a result of the manufacturing process and/or the source should be addressed as substances.
General (non-endpoint) information includes the chemical substance identity, information on related other datasets (i.e. Template, Mixture, Category), information on manufacture, use and exposure and information on classification and labelling. In the following, a brief overview of the IUCLID sections is given with cross references to more detailed descriptions, where appropriate.
Section 0: Related information
This section is actually not a data entry section, but provides means to relate other IUCLID elements to a dataset or indicates any related information.
In Substance datasets, the following subsections are provided:
Section 0.1 Templates: So-called Inherit Templates can be added to a dataset and are then indicated as links (i.e. Template datasets serving as containers for Endpoint records which are "inherited" to a dataset). In addition, Endpoint records can be copied from so-called Copy Templates. For detailed guidance, see chapter D.5 Template (Create and update Template related information).
Section 0.2 Categories: Links to all Categories are provided which the Substance dataset under consideration is related to as member of these chemical categories. For detailed guidance, see chapter D.6 Category (Create and update Category related information).
Section 0.3 Mixtures: Links to all Mixture datasets are provided which the Substance dataset under consideration is related to as a component.
In Mixture datasets, the following subsections are provided only:
Section 0.1 Templates: as above.
Section 0.4 Related Substances: Substance datasets related to the Mixture as components can be added and are then indicated as links. For detailed guidance, see chapter D.7 Mixture (Create and update mixture related information).
For more information, see chapter D.4.6 How to manage sections 0 - 3.
Section 1: General information
General information on the substance includes its chemical identity as represented by the associated Reference substance, its composition, its various business relationships (identity of sponsors, suppliers or recipients and members of a joint submission/consortium), identifiers assigned by regulatory programmes (e.g. REACH registration number) and other IT systems (e.g. IUCLID 4 reference), analytical information and spectral data, and information on product and process oriented research and development (if applicable). For more information, see chapter E.1 Section 1: General Information.
Section 2: Classification and labelling
The classification and labelling information can be added to this section according to the Globally Harmonised System for Classification and Labelling (GHS) and /or according to the European Directives (67/548/EEC for substances and 1999/45/EC for preparations) and amendments and adoptions thereof. For more information, see chapter E.2 Section 2: Classification and Labelling.
Section 3: Manufacture, use and exposure
Information stored in this section includes the following: information on the manufacturing methods, estimated quantities of production, import and use, production/use sites, availability in the supply chain (available as a substance as such, or in an article, or in a mixture), uses and exposure scenarios, waste production, and chemical compounds resulting from the production or use of the substance. For more information, see chapter E.3 Section 3: Manufacture, Use and Exposure.
Note
Exposure scenarios are sets of conditions that describe how substances are manufactured or used during their life-cycle and how the manufacturer or importer controls, or recommends downstream users to control, exposures of humans and the environment.
In REACH, if the Substance is classified as dangerous or is PBT or vPvB, then an exposure assessment and risk characterisation shall be performed to demonstrate that the risks are adequately controlled. This exposure assessment is done using exposure scenarios for each use of the substance.
In Substance or Mixture datasets, the term "endpoint" is used in the following meaning: An endpoint is an information requirement or data point with regard to the physico-chemical properties of the substance, environmental fate and behaviour, ecotoxicological information, toxicological information and specific information (e.g. effectiveness against target organisms or residues in food and feedingstuffs) according to a given chemical regulatory programme. In a wider sense, also additional information related to endpoints is included, i.e. guidance on safe use, information on literature search and a container section for attaching assessment reports.
In the context of REACH, IUCLID provides the format of the technical dossier referred to in Article 10(a), which shall include study summaries and robust study summaries of the information derived from the application of Annexes VII to XI of the REACH Regulation (EC) No 1907/2006. These study summaries comprise the endpoint data which are entered in Endpoint study records. They are the crucial elements in Endpoint sections. The principles including the key study / robust study summary approach are explained in the following subchapters.
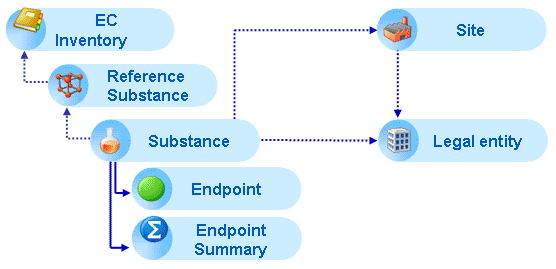
In addition, Endpoint summary records can be added throughout the section hierarchy for summarising the most critical results and conclusions of a given Endpoint section.
The following figure illustrates that Endpoint study and Endpoint summary records (solid lines) are part of the Substance dataset, while all other elements are referenced (dotted lines):
The IUCLID sections briefly described below are available for storing endpoint data. In these sections, context-sensitive online Help can be accessed for each data entry field (see chapter A.3 Online Help). In chapter E. Specific Guidance on Content of IUCLID Sections, these context-sensitive help texts are also listed for each Endpoint section.
Section 4: Physical and chemical properties
Data on physical and chemical properties such as melting point, boiling point, density, vapour pressure, etc. are stored in this section.
Section 5: Environmental fate and pathways
Data on stability, biodegradability, and/or bioaccumulation in different environmental compartments, transport and distribution information, and/or monitoring information, are stored in this section.
Section 6: Ecotoxicological information
Endpoint data on aquatic, sedimentation, and/or terrestrial toxicity, as well as endpoints on the monitoring of biological effects and biotransformation and degradation products, are stored in this section.
Section 7: Toxicological information
Endpoint data on toxicological information such as toxicokinetics, acute, repeated dose, genetic toxicity, carcinogenicity, toxicity to reproduction, etc. are stored in this section.
Section 8 Analytical methods
This section can be used to specify analytical methods for a substance in different matrixes or media (e.g. air, animal and human body fluids and tissues and soil).
Section 9 Residues in food and feedingstuffs
Information on residues in food or in feedingstuff, which is pesticide and possibly biocide specific, is stored in this section.
Section 10 Effectiveness against target organisms
This section can be used to enter information on the effectiveness against target organisms. It is specifically intended for biocides purposes.
Section 11: Guidance on safe use
In this specific IUCLID section, guidance on safe use can be provided, namely on first-aid measures, fire-fighting measures, accidental release measures, handling and storage, transport information, exposure controls / personal protection, stability and reactivity, disposal considerations.
Section 12: Literature search
In this section, details on any literature searches can be given.
Section 13: Assessment reports
This section is not provided for entering information in data entry fields, but to attach assessment reports. See chapter B.4.3. Assessment reports.
In this manual, the following definition is used for the term "study summary":
The term "study summary" stands for any summarising description of a full study report.
A full study report can either concern an experimental study as available from company files or from the published literature (regardless of whether the data have been generated in accordance with Good Laboratory Practice guidelines or to the latest test guideline method), information derived from an estimation or prediction method (e.g. based on QSAR, SAR models), information available from surveys, handbooks, review papers, monographs, criteria documents, etc., and any other information that is or may be relevant for a given information requirement.
Study summaries can be very condensed or very detailed. In other words, the term "study summary" is not indicative of the documentation completeness in terms of the most relevant study elements. However, a study summary should provide sufficient information to make an assessment of the relevance of the study.
Very detailed study summaries are also termed "robust study summaries", if they address all relevant study items, as further outlined below (see chapter B.4.2.2.3.1 Definition of key studies, supporting studies, robust study summaries).
This definition is in line with that given in REACH, Title I, Article 3(29): "Study summary: means a summary of the objectives, methods, results and conclusions of a full study report providing sufficient information to make an assessment of the relevance of the study."
In IUCLID, study summaries are managed using Endpoint study records as outlined in chapter D.4.7 How to manage Endpoint study records in sections 4 - 13.
The first step in fulfilling the information requirements set out by a regulatory programme involves the gathering of existing information. For example, the REACH regulation makes it clear that all information should be considered. That is, not only the information requirements referred to in Article 10, but all other available and relevant information on the substance regardless whether testing for a given endpoint is required or not at the specific tonnage level.
The different categories of information that can to be summarised in IUCLID Endpoint records, as laid down in Annex XI of REACH, include:
Studies conducted in accordance with Good Laboratory Practice guidelines and standardised test methods, which include, for EU regulations, the test methods laid down in a Commission Regulation or in accordance with other international test methods recognised by the Commission or the European Chemicals Agency as being appropriate.
Existing data, provided that they are relevant and reliable:
Data on physical-chemical properties from experiments not carried out according to GLP or recognised test methods
Data on human health and environmental properties from experiments not carried out according to GLP or recognised test methods
Historical human data
Weight of evidence evaluation of the available information, if it allows to judge the presence or absence of a particular substance-inherent (e.g. a particular dangerous) property.
Qualitative or Quantitative structure-activity relationship ((Q)SAR), if it allows to judge the presence or absence of a particular substance-inherent (e.g. a particular dangerous) property.
Grouping of substances and read-across approach, when particular intrinsic properties of a substance can be predicted by interpolation from data available for another substance or other substances within the group.
In vitro methods, if they are sufficiently well developed according to internationally agreed test development criteria (e.g. the European Centre for the Validation of Alternative Methods (ECVAM)) and if the results may indicate the presence of a certain dangerous property or may be essential in relation to a mechanistic understanding, which may be important for the assessment.
Justifications and documentations that indicate that testing for specific endpoints is technically not possible.
Justifications and documentations that indicate that testing for specific endpoint are not needed, based on exposure scenario(s) developed.
In IUCLID Endpoint study records, several elements are provided towards the adequate documentation of either of above categories of information. These include for example flag fields for specifying the study result type (e.g. "experimental study" or "read-across based on grouping of substances (category approach)") or fields for claiming and justifying data waiving. Guidance on how to use these elements is given in chapter D.4.7.7.1 Administrative data.
The terms "key study" and "robust study summary" have sometimes been used as synonyms. Although it is true that a key study is normally summarised in the form of a robust study summary, the reverse conclusion does not always apply. As outlined below, it can be required to prepare robust summaries even for non-key studies.
"Key studies" can be defined as follows:
A key study is generally expected to be the most adequate, reliable and relevant study for a specific element or, in the case of IUCLID, for a specific endpoint study section.
A key study should be described in compliance with the definition of robust study summaries, that is, summaries of key studies provide a reasonable amount of details.
IUCLID is not prescriptive as to how many key studies are required for each data requirement or how detailed they should be described. Refer to the relevant guidance for the respective regulatory programme thereon.
The term "supportive studies" or "supporting studies" refers to other adequate studies that are considered "supportive" of the key study or key studies. The level of detail to be used for the description of supporting studies is usually to be decided on a case-by-case basis. For instance, detailed descriptions can be sensible if these studies are used to defend the key study identified against conflicting results from less valid studies.
For key and other relevant studies, various regulatory programmes require study summaries that have a certain degree of "robustness". Robust study summaries are characterised as follows:
They include as much technical information as necessary to adequately describe an experiment or study.
Hence, robust study summaries adequately reflect the objectives, methods, results, and conclusions of the full study report.
The term "robust" comes from the fact that an exhaustive description of the various study items or parameters is necessary together with evaluative comments made by the data reviewer.
The objective of robust study summaries is to provide sufficient information to allow a technically qualified person to independently assess a given study report without having to go back to the full report, and to also allow evaluation of the proposed test plan.
This definition is in line with that given in REACH, Title I, Article 3(28): "Robust study summary: means a detailed summary of the objectives, methods, results and conclusions of a full study report providing sufficient information to make an independent assessment of the study minimising the need to consult the full study report."
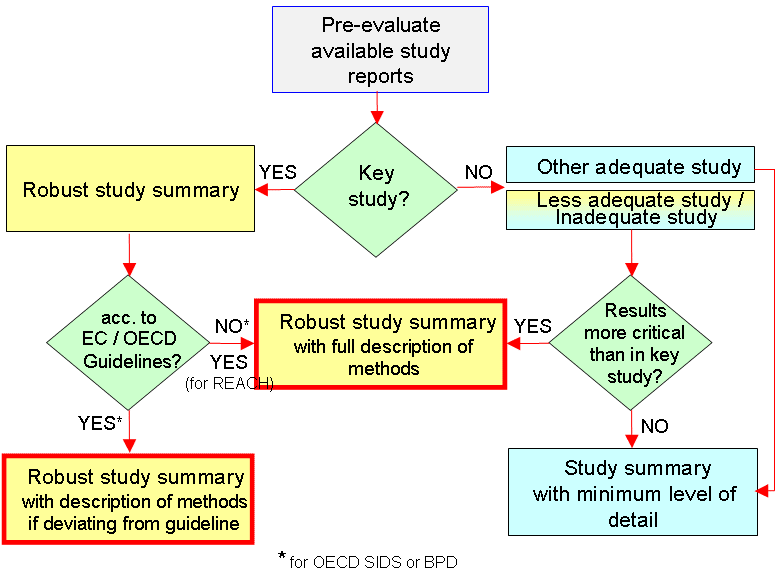
In dossiers compiled in the context of EU REACH, EU BPD or OECD SIDS, robust study summaries are of particular relevance for the adequate presentation of any study that is or could possibly be relevant for hazard or risk assessment. This is in general true for "key studies". However, other studies may also require detailed descriptions if they are of possible relevance. In particular, for studies that are flawed, but indicate critical results, robust study summaries highlighting the weaknesses of the studies need to be prepared as well.
The following graph as adapted from guidance documents for OECD SIDS* and EU BPD** has been slightly modified to account for REACH requirements. It shows a decision tree for defining the level of detail:
Important
As to the requirements on robust study summaries refer to the relevant guidance for the respective regulatory programme. For example, following requirements are laid down in the relevant guidance documents for REACH and BPD, respectively:
REACH: The full description of methods is strongly recommended for all robust study summaries.***
BPD: The TNsG on Data Requirements** requires to detail also studies with "positive" findings for the endpoints mutagenicity, carcinogenicity and teratogenicity.
* OECD 2004: OECD Manual for Investigation of HPV Chemicals.
** ECB 2002: Technical Guidance Document in support of the Directive 98/8/EC concerning the Placing of Biocidal Products on the Market - Guidance on Data Requirements for Active Substances and Biocidal Products.
*** Consult the relevant Guidance on Registration documents prepared by the European Commission in the context of the REACH Implementation Projects (RIPs).
The user interface of the endpoint study sections of IUCLID has been designed in such a way that the key / robust study summary approach is fully reflected:
There are flag fields for indicating if the study recorded is a key or supporting study and if it is summarised in the form of a robust study summary.
All data entry fields are defined as being relevant for either "basic data" or "additional data". The latter can be hidden or displayed as to the user's convenience as further described in chapter D.4.7.6 Switching between display type "basic fields" (detail level 1) and "all fields" (detail level 2).
The following levels of detail are currently used in IUCLID:
This detail level includes all basic fields as defined in the OECD harmonised templates. These fields should be completed for each study summary as far as possible. However, depending on the type of information and the information source even some of these basic fields cannot always be filled in.
This detail level comprises all fields, i.e. basic and additional fields as defined in the OECD harmonised templates. On the IUCLID interface, the labels of additional fields are set in blue fonts, while the labels of basic fields are black. Completion of additional fields is normally only necessary for robust study summaries.
Caution
For technical reasons, all subfields grouped in a block of fields are assigned to a given detail level, i.e. either 1 or 2. However, some repeatable blocks of fields contain fields that are actually considered as additional fields and hence, only relevant for robust study summaries. Refer to the context-sensitive online Help provided by pressing the F1 key from within any field or clicking the HELP icon on the menu bar.
Note
In the OECD harmonised
templates, the field Confidential details on test
material is assigned to the detail level 3 with the intention to
have the option of filtering out this field from export, print or dossier
files as appropriate. In IUCLID, this option is indeed be offered in the
dialogue of any of these operations, though this field is not explicitly
indicated as detail level 3 field on the user interface.
In the endpoint-related IUCLID sections 4 to 10, endpoint summary templates are provided at various locations in the section hierarchy, e.g. for 6 Ecotoxicological Information, 6.1 Aquatic toxicity, 6.1.1 Short-term toxicity to fish.
An endpoint summary includes the summary of the evaluation made of all relevant (robust) study summaries compiled in a specific IUCLID section. On the endpoint level (e.g. 6.1.1 Short-term toxicity to fish), an endpoint summary should focus on the most important information, i.e. most critical results and conclusions, and justify the use of certain studies for this hazard identification. This information can come directly from a key study, but it can also be derived form a weight of evidence approach or any other means (e.g. a mean value for a physico-chemical parameter).
The purpose of an endpoint summary is to
reflect and evaluate all available information for a specific endpoint in the technical dossier;
use them for the chemical safety report, if required according to the REACH regulation, or for similar risk assessment documents in other regulatory programmes. As far as feasible, an automatic transfer of endpoint summaries to such documents may be done.
On a higher level of the IUCLID section hierarchy, endpoint summaries provide the possibility to document elements that are needed for the chemical safety assessment. These include:
Predicted no-effect concentrations (PNECs) for environmental compartments including a justification of the PNEC derivation and the assessment factors used (section 6 Ecotoxicological Information);
Derived no-effect levels (DNELs) for workers and the general population (including consumer exposure) including a justification of the DNEL derivation and the assessment factors used (section 7 Toxicological information);
The physico-chemical properties of a substance which may cause risks such as explosivity, flammability and oxidising potential (section 4 Physical and chemical properties).
Detailed guidance is given in chapter D.4.8 How to manage Endpoint summary records in sections 4 - 10.
IUCLID provides the tools to manage different dossiers and other reports as required for specific regulatory programmes. A Dossier in general is a collection of information about a chemical substance or grouped substances generated towards a particular purpose. A Dossier is prepared by a body that is so obliged according to a given legislation and this can be a registrant, a notifier, an applicant for authorisation or a regulatory agency evaluating these submissions.
A IUCLID Dossier represents a particular view on the data compiled in dataset(s). For instance, an OECD SIDS dossier includes all data elements, i.e. sections, relevant for this type of dossier, while a REACH registration dossier includes all company and substance data elements required in accordance with Article 10 of the REACH regulation. In general, a IUCLID Dossier can be characterised by the following criteria:
A IUCLID Dossier is a copy of the selected data elements from the raw data (i.e. datasets where the data are entered and edited), the selection being performed according to a specific Dossier template. The Dossier does not have to include all data elements defined in the template, the user being free to select whichever elements he/she desires.
A IUCLID Dossier is a read-only snapshot of a dataset, i.e. of the raw data.
Any recipient of a Dossier must also receive and import the related raw data in order to be able to edit these data.
Dossier data can be copied to a dataset, but cannot be edited in that dataset.
There is one special Dossier template available, which encompasses all possible data elements. This so-called Complete Dossier template allows tailoring the data elements to be included in the Dossier to any requirements, for which no specific Dossier template is provided. Apart from this generic template, the following legislations or chemical programmes are supported by specific IUCLID Dossier templates:
OECD
OECD SIDS: SIDS Dossiers for HPV Chemicals
EU BPD (Directive 98/8/EC concerning the placing of biocidal products on the market)
Biocides - Active ingredients
Biocides - Biocidal products
Biocides - Substances of concern
EU REACH (Regulation (EC) No 1907/2006)
Dossiers or reports related to specific submissions by industry
REACH C&L notification
REACH C&L notification member of a joint submission
REACH Notification of substance in article
REACH PPORD (Product and process orientated research and development)
REACH Registration 1 - 10 tonnes, physicochemical requirements
REACH Registration 1 - 10 tonnes, standard requirements
REACH Registration 10 - 100 tonnes
REACH Registration 100 - 1000 tonnes
REACH Registration above 1000 tonnes
REACH Registration member of a joint submission - general case
REACH Registration member of a joint submission - intermediates
REACH Registration on-site isolated intermediates above 1 tonne
REACH Registration transported isolated intermediates 1 - 1000 tonnes
REACH Registration transported isolated intermediates above 1000 tonnes
REACH Application for authorisation
Dossiers generated by the European Chemicals Agency or Competent Authorities of the Member States
REACH Substance Evaluation
REACH Annex XV - Authorisation
REACH Annex XV - C&L harmonisation
REACH Annex XV - Restriction
Other
The following template may be used in special cases for including only Endpoint sections in a Dossier:
Endpoints information
Matrix reports for chemical categories
The IUCLID feature Category includes a tool for generating a Matrix Report. This is a very condensed report of all data elements for which a chemical category has been defined. The main purpose is to provide an overview of the data availability and a rapid and easy comparison of all relevant endpoints for all category members. For detailed instructions on the category approach and how to manage it in IUCLID see chapter D.6 Category (create and update Category related information).